Figures & data
Figure 1 Recovered xenografts. (A) An index severed in zone II from a hand laborer, which did not fulfill conditions allowing microsurgical revascularization. (B) A stiff finger that hindered functioning of the hand was removed during a scheduled operation.
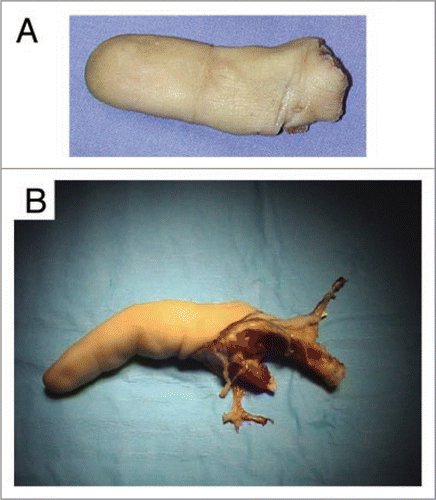
Figure 2 After recovery of the finger, the dominant palmar collateral artery was catheterized and perfused abundantly with Custodiol® solution until flow through from the vein was clear.
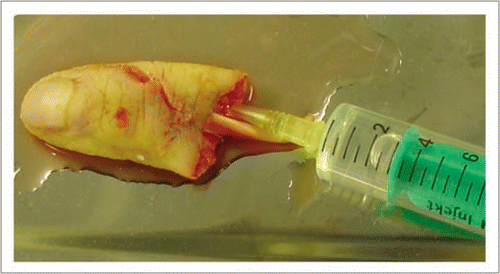
Figure 3 Double walled container “Cryokit®” used for packaging the xenotransplant allowing a safe and constant hibernation during transport.

Figure 4 After intra-arterial administration of the cryoprotector, the finger was placed in a specially adapted, three-vent bag and immersed in the cryoprotective solution with no air bubbles.

Figure 5 The rabbit was used as an animal model. Xenograft implantation was performed in the cervical region with vascular attachment to the carotid artery and the jugular vein.
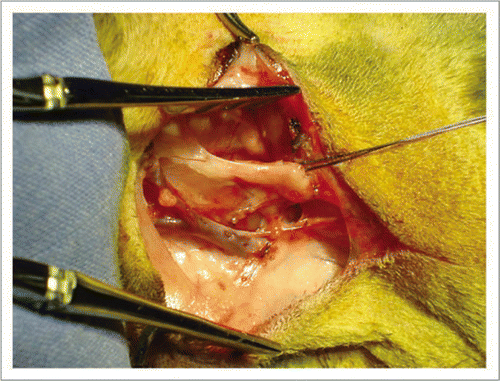
Figure 6 The dominant collateral artery was again catheterized and the finger abundantly rinsed with physiological serum until tissues recovered a normal consistency. Heparin was administered intravascularly to avoid non-reflow phenomenon.
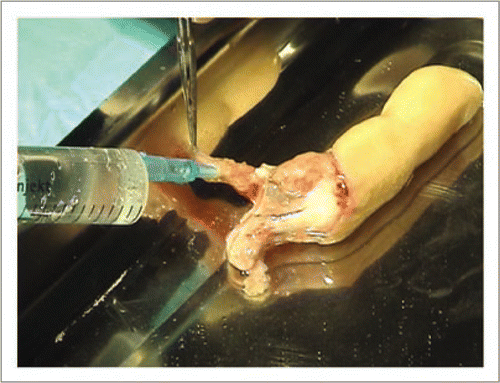
Figure 7 Following microsurgical vascular attachment, the xenograft was positioned around the neck and secured with sutures at both extremities.
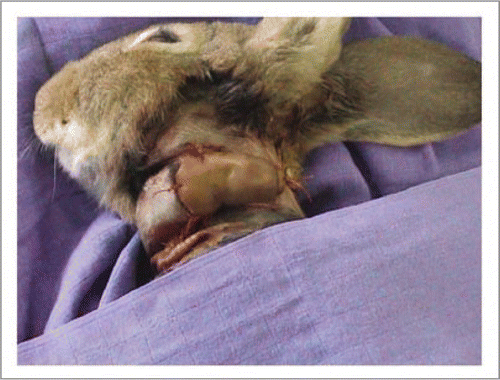
Figure 8 The rabbit was placed in a cage with its head immobilized to avoid inopportune movement. A perfusion was placed in the auricular vein to administer anticoagulants and fibrinolytics.
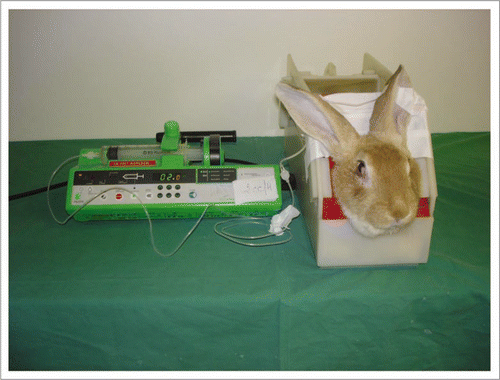
Figure 9 (A) End-to-end arterial attachment of the dominant palmar artery of the finger to the rabbit's carotid artery. (B) Upon release of the arterial clamps, xenograft revascularization was immediate, with appearance of a venous flow-through. However, the finger became rapidly marbled and the capillary pulse was hardly perceptible.
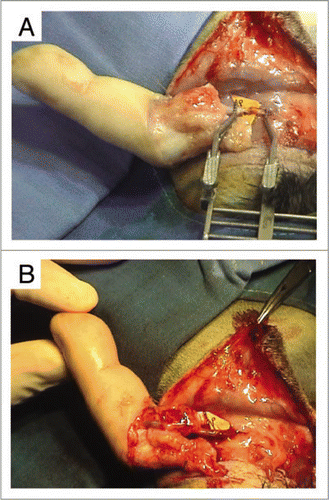
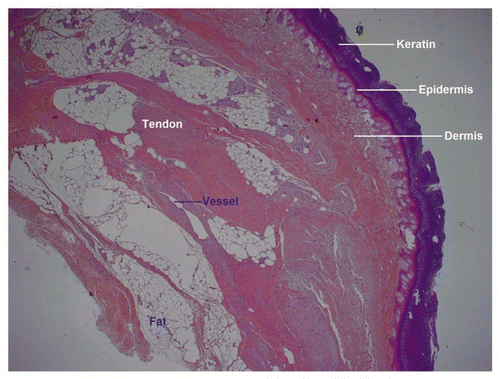
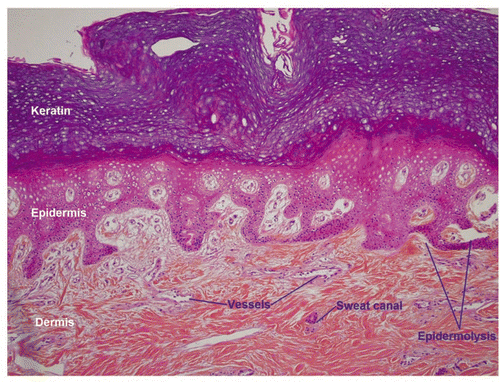
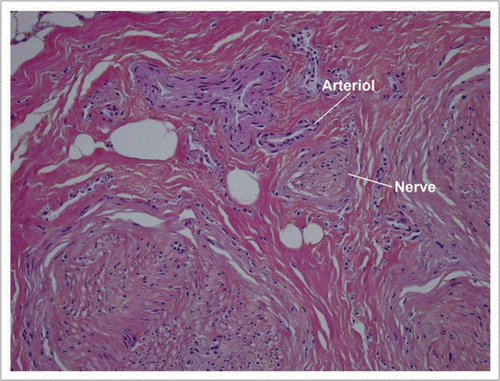
Figure 10 Low power histology sections showed that cutaneous, hypodermal, tendon, neural and vascular tissues retained their normal architectures.