Figures & data
Figure 1 p27Xic1 is expressed at late tail bud stages in the pronephros by in situ analysis. Whole mount in situ hybridisation was carried out with a DIG-labelled anti-sense RNA probe for p27Xic1. Prior to and including stage 25, p27Xic1 expression in the presumptive pronephros is not detected by in situ hybridisation. At stage 27 p27Xic1 expression is clearly evident in the dorso-anterior region of the presumptive pronephros (white arrow). Pronephric staining is confirmed in transverse sections at stage 27 through the anterior pronephros anlagen (white arrow). At stage 30 a similar pattern of p27Xic1 expression to that seen at stage 27 is observed in the dorsal anterior pronephros anlagen. By stage 32 p27Xic1 expression is concentrated in the nephrostomes and proximal tubules. No pronephric expression of p27Xic1 is detected by in situ hybridisation at stage 38. (som, somites; nt, neural tube; ba, branchial arches; pr, pronephros; lh, lymph heart; ma, migrating muscle anlagen).
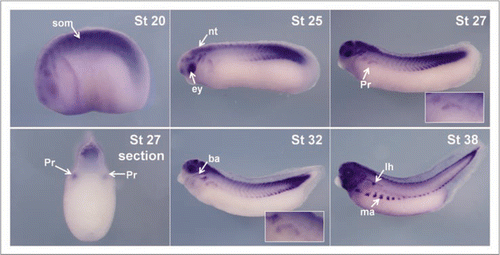
Figure 2 Overexpression of p27Xic1 identifies a pronephric phenotype. X. laevis embryos were injected at the 8 cell stage into a V2 blastomere to target the somites and presumptive pronephric region. mRNA of the construct indicated was co-injected with βgal mRNA to act as a lineage tracer (stained blue, black arrow). Embryos were cultured to stage 41 and whole mount antibody stained with 3G8, to detect proximal pronephric tubules (white arrow, stained purple), and 4A6, to detect intermediate and distal pronephric tubules (white arrowheads, stained red). Control βgal mRNA injected embryos had normal pronephros development (A). p27Xic1 mRNA and p21Cip1 mRNA injections inhibited proximal, intermediate and distal tubule development on the injected side (B and C). p27Xic1 N mRNA reduced the size of the pronephros, as indicated by reduced 3G8 and 4A6 staining (D). p27Xic1 #2 mRNA, p27Xic1 C mRNA and p27Xic1 CK− mRNA injections had no effect on pronephros development (E–G). These results are displayed in a histogram (H). *denotes injected side.
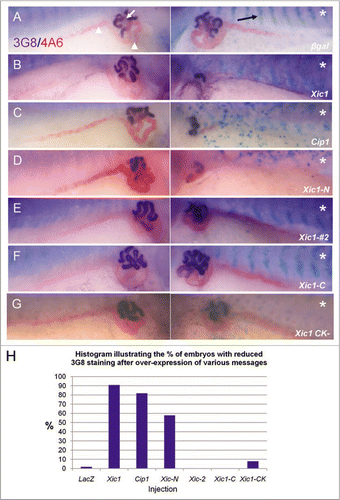
Figure 3 Inhibition of endogenous p27Xic1 mRNA translation using a specific MO identified a pronephros phenotype. X. laevis embryos were injected at the 8 cell stage into a V2 blastomere to target the somites and presumptive pronephric region. βgal mRNA was co-injected to act as a lineage tracer (blue staining in B–D and red staining in E–G). Embryos were cultured till stage 32, where they were whole mount in situ hybridised for nephrin expression (a marker of the glomus, A–C), or stage 41, where they were whole mount antibody stained with 3G8 and 4A6 to detect tubules (D–F). Control MO injected embryos had normal glomus development (A) and tubulogenesis (D). Injection of p27Xic1 MO inhibited formation of the glomus (B) and proximal, intermediate and distal tubules on the injected side (e). Overexpression of p27Xic1 also inhibited nephrin expression (C). p21Cip1 mRNA almost completely rescued development of the pronephros when co-injected with the p27Xic1 MO (F). *denotes injected side.
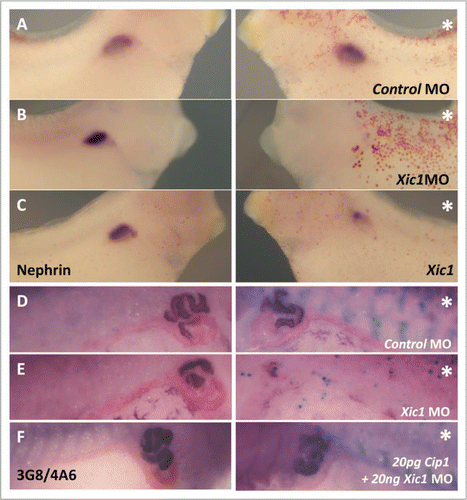
Figure 4 p27Xic1 overexpression and depletion, using a MO, inhibited pronephros anlagen formation with the same severity in the presence or absence of the apoptotic inhibitor BclXL. X. laevis embryos were injected at the 8 cell stage into a V2 blastomere to target the somites and presumptive pronephric region. βgal mRNA was co-injected to act as a lineage tracer (stained red, white arrow). embryos were cultured till stage 22 and whole mount in situ hybridised for expression of Lim-1, an early marker of the pronephros anlagen. Injection of the Control MO had no effect on Lim-1 expression (A). p27Xic1 mRNA, p27Xic1 MO, p27Xic1 mRNA/1 ng BclXL mRNA, and p27Xic1 MO/1 ng BclXL mRNA all reduced expression of Lim-1 on the injected side (B–E). Injection of p27Xic1 mRNA and p27Xic1 MO had effects on muscle development (see section 2.9); hence curling of the embryos towards the injected side was frequently observed, such as in the p27Xic1/BclXL injected embryo shown here (E). *denotes injected side.
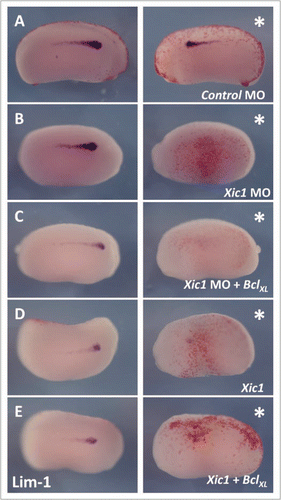
Figure 5 Overexpression of p27Xic1 mRNA reduced cell division, whereas depletion of endogenous p27Xic1 mRNA translation using a MO had no affect on cell division. X. laevis embryos were injected into one cell of a two cell stage embryo with βgal mRNA to act as a lineage tracer (red staining). Embryos were cultured till stage 22 and whole mount in situ hybridised for expression of Lim-1, and antibody stained for phosphohistone H3 (pH3, a marker of dividing cells). The Control MO had no effect on Lim-1 expression (white arrow) or pH3 immunostaining (white arrowhead) (A). p27Xic1 mRNA reduced both Lim-1 expression and pH3 immunostaining (B). p27Xic1 MO had no effect on pH3 immunostaining but reduced Lim-1 expression (C). p27Xic1 CK− mRNA had no effect on either Lim-1 expression or pH3 staining (D). *denotes injected side. To quantify the effect of these injections on cell division, positively pH3 immunostained cells on the injected and un-injected side of the embryos were numerically scored. The differences in the number of pH3 immunostained cells between the injected and un-injected sides were calculated as percentages and are presented as a bar chart (E). The Control MO, p27Xic1 MO and p27Xic1 CK− mRNA had no statistically significant effect on pH3 immunostaining. p27Xic1 mRNA statistically significantly reduced pH3 immunostaining on the injected side on average by 63% when compared to the un-injected side.
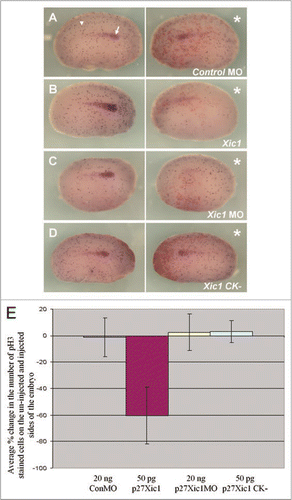
Figure 6 Mis-expression of p27Xic1 disrupted somite morphology and muscle differentiation, but not early mesoderm formation.to observe the effects p27Xic1 depletion had on early mesoderm formation, we injected both cells of a 2-cell stage embryo with the p27Xic1 MO, cultured the embryos to stage 10/11 and performed whole mount in situ hybridisation for Xbrachyury, a marker of the early mesoderm. Injection of neither the Control MO or the p27Xic1 MO had any effect on Xbrachyury expression (A and B).to observe the effects of p27Xic1 mis-expression on myogenesis, X. laevis embryos were injected at the 8 cell stage into a V2 blastomere to target the presumptive pronephric region. βgal mRNA was co-injected to act as a lineage tracer (red staining). embryos were cultured till stage 24 and whole mount in situ hybridised for expression of both Lim-1 and MyoD (a marker of differentiating muscle). The Control MO had no effect on either Lim-1 (as marked by the white arrowhead) or MyoD expression (as marked by the white arrow) (C). p27Xic1 MO reduced expression of both Lim-1 and MyoD (D). p27Xic1 mRNA reduced Lim-1 expression and disrupted MyoD expression (dashed white arrow), causing non-segmentation of the somites (E). p27Xic1 CK− had no effect either Lim-1 or MyoD expression (F). *denotes injected side.
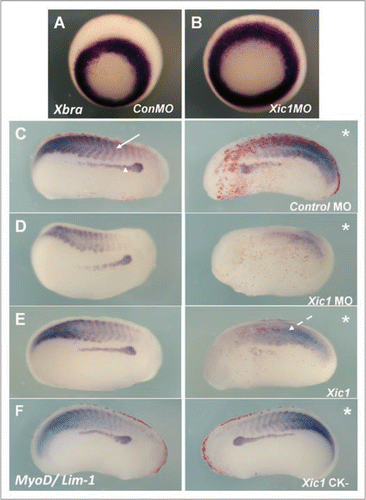
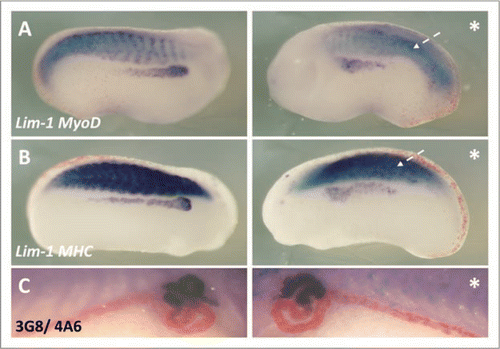
Figure 7 HUA treatment severely perturbed pronephric and muscle development. Stage 10.5 X. laevis embryos were incubated in either HUA and DMSO or DMSO only (control) and left to develop to stage 41 where whole mount 3G8/4A6 antibody staining was performed to detect the mature pronephros. All embryos treated with HUA had delayed development and reduced 3G8/4A6 staining (arrowheads). The control embryo showed normal development at stage 41 (A). Stage 10.5 X. laevis embryos were incubated in either HUA and DMSO or DMSO alone (control) and left to develop to stage 23 where whole mount in situ hybridisation for Lim-1/MyoD (B) and Lim-1/MHC (C) expression was performed. The HUA treated embryo is shown above the control untreated embryo in (B and C). HUA treatment clearly inhibited pronephros anlagen formation, as seen by reduced Lim-1 expression (white arrow), and disrupted the normally segmented MyoD expression (B) (white arrowhead) and MHC expression (C) (black arrowhead).
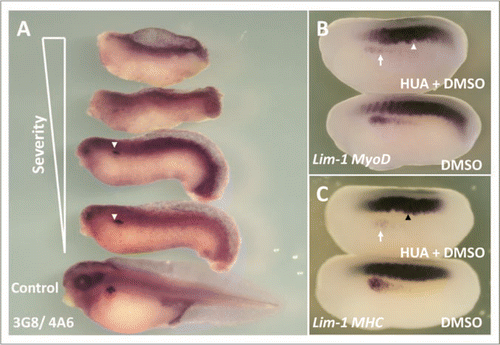
Figure 8 p35.1 disrupted somitogenesis and pronephros anlagen formation, but not mature pronephros development. X. laevis embryos were injected at the 8 cell stage into a V2 blastomere to target the presumptive pronephric region. βgal mRNA was co-injected to act as a lineage tracer (red staining). Embryos were cultured till stage 24, where whole mount in situ hybridised for expression of Lim-1/MyoD (A) and Lim-1/MHC (B) was carried out, and to stage 41, where whole mount 3G8/4A6 antibody staining was performed. p35.1 mRNA overexpression disrupted development of the myotome and pronephros anlagen (A and B). Both MyoD and MHC expression were abnormal on the injected side, with segmentation of the somites apparently lacking. Lim-1 expression was in a broader domain on the injected side. However at stage 41 p35.1 mRNA overexpression had no effect on the size of the mature pronephros (C). In this image the lineage tracer is not lined up in the typical chevron pattern associated with appropriate segmentation, indicating to us the early effects on somitogenesis have not recovered. *denotes injected side.