Figures & data
Figure 1 Photomicrographs of (A and C) control and (B and D) HUCPVC-containing wounds at 7 days. Note in (A) the size of the original circular wound and with annular repair and central eschar. (B) The HUCPVC-treated wound shows more advanced repair. (C and D) Wounds viewed by transillumination show little vascularization in the control (C) but marked radial arrays of blood vessels in the HUCPVC treated wound (D). Bars = 2 mm.
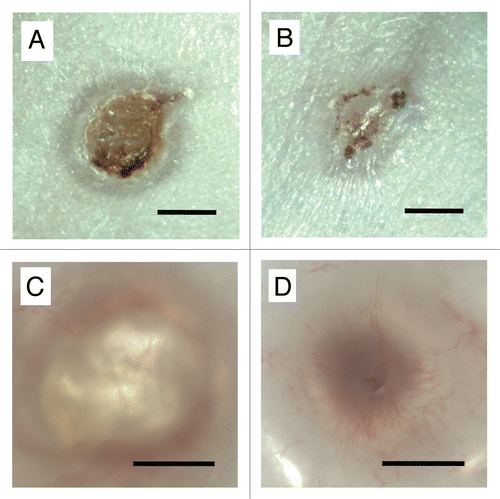
Figure 2 Sections of 4 mm diameter defects at 3 days. (A) Fibrin control, note poorly organized dermis. (B) HUCPVC in fibrin, note the obvious presence of cells within the fibrin and the resulting maintenance of dermal thickness. Field width for each image: 858 µm.
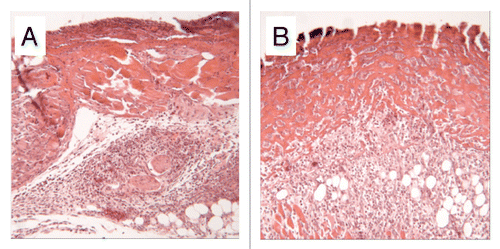
Figure 3 Photomicrographs of cryosections of (A) control and (B) HUCPVC-treated defects at 7 days showing incomplete re-epithelialization in the control and complete re-epithelialization in the HUCPVC specimen. Note the prominent microvasculature in (B). (C and D) show a pair of serial cryosections of a HUCPVC-treated defect at 7 days. (C) stained with Toluidine blue, shows the general tissue morphology with rete ridge formation, while the epifluorescent image in (D) shows the localization of the HUCPVC in the dermis. Field widths: (A and B) 2,144 µm, (C and D) 858 µm.
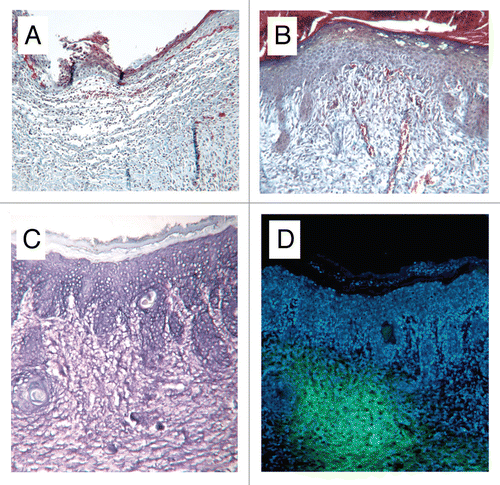
Figure 4 Fluorescence microscopy of cultures obtained from wound digests. (A) Murine cells derived from digestion of control defects, no presence of PKH67. (B) Populations of PKH-67-stained HUCPVCs were identified alongside murine-derived cells following digestion of treatment defects. Intact DAPI-stained nuclei indicate viable cells. (C) Immunocytochemical analysis for Ki-67 (red, visualized using Texas red) colocalized to DAPI-stained cell nuclei of PKH67-positive (green) HUCPVCs. Field widths: (A and B) 29 µm, (C) 214 µm.
Figure 5 Individual wound tensile strength measurements of 8 mm diameter full thickness circular defects 7 days post-operative. For each animal (numbered 1–6) pink bars represent single tensile measurements and blue diamonds represent mean wound tensile strength.
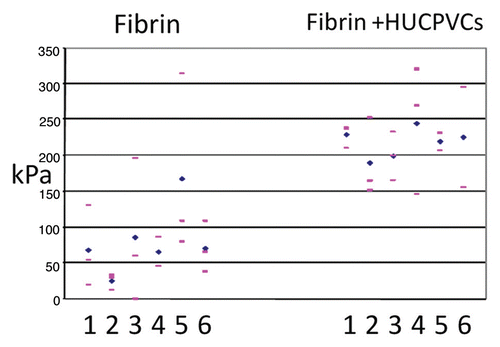
Figure 6 Mean wound tensile strength (kPa) of 8 mm diameter full thickness circular defects at 3, 7 and 10 days postoperative compared with normal skin. Bars represent standard deviations for each value.
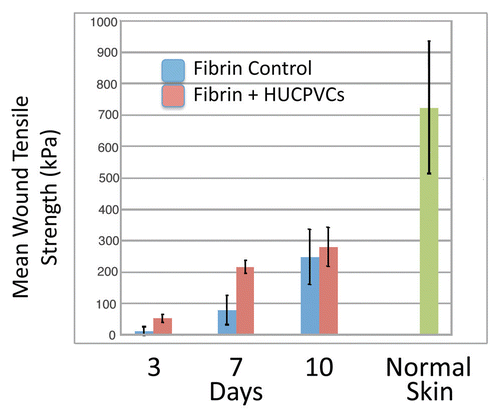
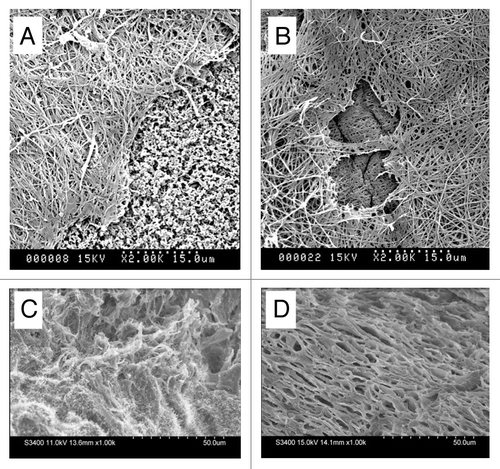
Figure 7 Scanning electron microscopy of (A and B) diffusion chambers innoculated with HUCPVCs and implanted intraperitoneally in rats for 4 and 7 days respectively. A fibrillar extracellular matrix is evident at both time points. The exposed cellulose acetate filter (f) seen at 4 days but not at 7 days suggests continued HUCPVC synthetic activity in vivo to 7 days. (C and D) Freeze fractured 4 mm diameter full thickness circular defects at 7 days post-operative (C) from fibrin-only control wound; (D) from fibrin + HUCPVC wound. Note, in the latter, the organized stratified appearance of the ECM.