Figures & data
Figure 1 Molecular discrimination of H-type and typical BSE in cattle and in C57Bl/6 infected mice. Western blot detection of PrPres in cattle (A–C) or C57Bl/6 infected mice (E–G), from H-type isolate (right lane in each panel) or typical BSE (left lane in each panel), using 12B2 (A and E), Sha31 (B and F) or SAF84 (C and G) monoclonal antibodies. In each Western blot, typical BSE is shown in the left lane and H-type BSE in the right lane. Brain equivalent quantities loaded per lane were 100 µg (left lanes) and 250 µg (right lanes) for cattle samples and 1.2 mg (left lanes) and 4.8 mg (right lanes) for mouse samples. Inset of (A) shows the N-terminal PrPres fragment (≈7 kDa) in cattle (left lane) and mouse (right lane). (D) Interpretation of the Western blot profile observed with SAF84 monoclonal antibody. (H) Unspecific immunoreactivity of anti-mouse Ig conjugate on samples prepared from normal brain of C57Bl/6 mouse.
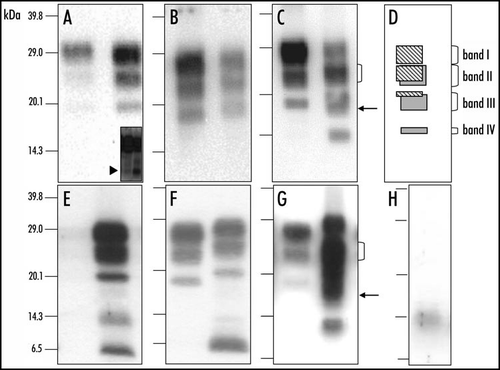
Figure 2 Molecular analysis of PrPres after deglycosylation in C57Bl/6 infected mice. Western blot analysis of PrPres from cattle (A–C) or C57Bl/6 mice (D–F) infected with H-type (lanes 3 and 4) or typical BSE (lanes 1 and 2), after deglycosylation by PNGase treatment. Monoclonal antibodies used for PrPres detection were 12B2 (A and D), Sha31 (B and E) or SAF84 (C and F). Brain equivalent quantities loaded per lane were (i) from cattle, for typical BSE, 50 (lane 1) or 2,6 (lane 2) mg, and for H-type BSE, 50 (lane 3) or 6 (lane 4) mg, and (ii) from mice, for typical BSE, 0.5 (D and F) or 0.3 (E) mg, and for H-type BSE, 2.5 (D and F) or 1.3 mg (E).
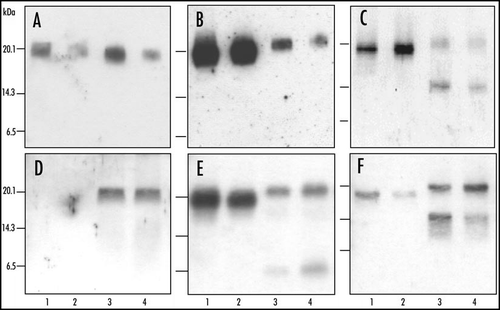
Figure 3 Quantitative molecular features of PrPres in C57Bl/6 infected mice. Molecular masses (A) and glycoform ratios (B) (means ± standard deviations) of PrPres detected with Sha31 antibody, from C57Bl/6 mice infected with H-type or typical BSE isolates. PrPres of mice infected with an experimental scrapie (C506M3) strainCitation4 was used as a control.
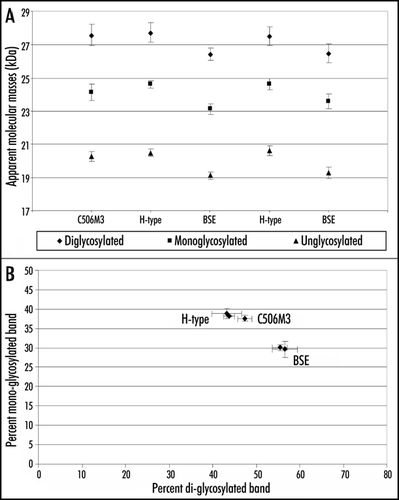
Figure 4 Schematic representation of proteinase K-resistant PrP fragments identified from H-type or typical BSE. Putative regions for proteinase K cleavage are indicated, as assessed by immunoreactivities with anti-PrP antibodies, and categories of epitope locations (groups A, B and C).
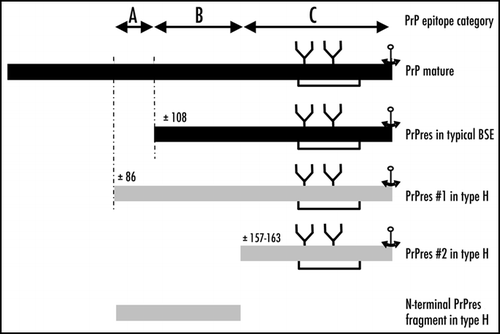
Table 1 Antibodies used to characterize H-type and typical BSE