Figures & data
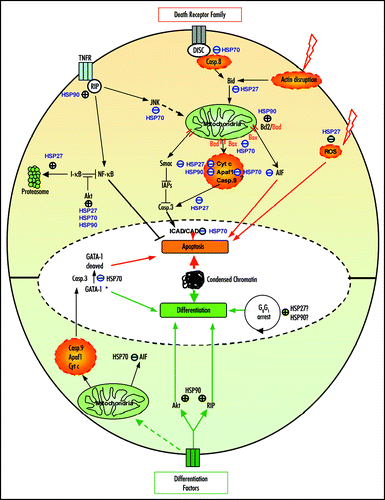
Figure 1 Modulation of apoptosis and differentiation by HSP90, HSP70 and HSP27. In apoptosis (upper part), HSP90 can inhibit caspase (casp.) activation by its interaction with Apaf1. HSP90 stabilizes proteins from the survival signaling including RIP, Akt and Bcl-2. HSP70 can block apoptosis by inhibiting JNK, Bax mitochondrial translocation, by interacting with AIF and/or Apaf-1 or by protecting essential nuclear proteins from caspase-3 cleavage. HSP27 can block caspase activation through its action on F-actin, Bid, ROS or cytochrome c. HSP27 also favors the proteasomal degradation of proteins like I-kBa. During differentiation (lower part), HSP90 stabilizes proteins involved in signaling induced by differentiating factors like Akt, RIP as well as Rb. HSP70 prevents the cleavage of GATA-1 by caspase-3 allowing erythoblast to differentiate instead of dying by apoptosis. HSP70 neutralization of AIF contributes also to the survival of the differentiating cells. HSP27 might be involved in the cell cycle arrest (→, stimulation; ⊥, inhibition; *, in erythroblasts).