Figures & data
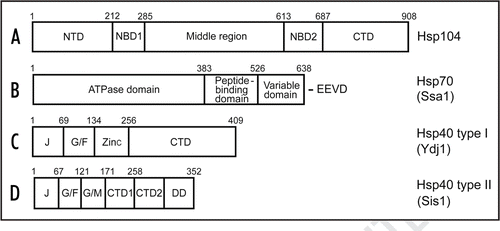
Figure 1 Structural organization of the yeast Hsp proteins involved in stress protection and prion propagation. (A) Hsp104; (B) Ssa1 as a representative of the Hsp70 family; (C) Hsp40 type I (Ydj1); (D) Hsp40 type II (Sis1). (The Hsp40 type III is not shown.) Designations: NBD, nucleotide-binding domain; NTD, N-terminal domain, middle region; CTD, C-terminal domain; J, J-domain; G/F, glycine and phenylalanine-rich region; Zinc, zinc-finger domain; G/M, glycine and methionine-rich region; DD, dimerization domain; E, glutamic acid; V, valine; D, aspartic acid. Numbers correspond to aa positions.
![Figure 2 Model for the role of molecular chaperones in formation and propagation of the [PSI+] prion. CSK, cytoskeleton; UPS, ubiquitin-proteasome system; IBs, inclusion bodies. See comments in text.](/cms/asset/6f25ab4f-60ed-4f17-a62c-f3b866ab96d9/kprn_a_10905058_f0002.gif)