Figures & data
Table 1. TSE diseases
Figure 1. PrPc is strongly expressed on FDCs in the lymphoid follicles of the spleen. Images taken from mouse spleen immunolabelled with the anti-CD45R (green, A), anti-PrP (1B3) (blue, B), and anti-CD35 (red, C) antibodies. FDCs and CD35-expressing B cells detected with the anti-CD35 specific antibody. B cells detected with the anti-CD45R specific antibody and PrPc expression was detected using the 1B3 polyclonal antibody.Citation126 (D) Merged image of all three antibodies.
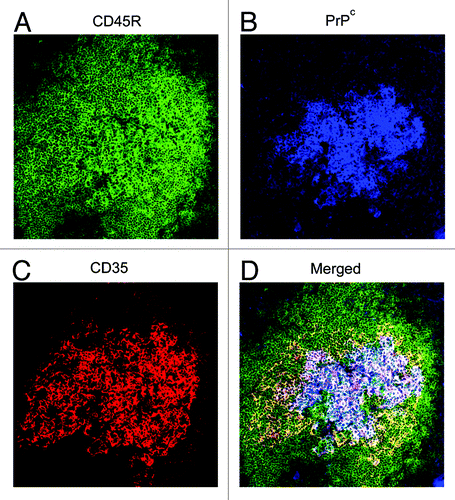
Figure 2. PrPSc accumulates in the draining LN following scrapie infection via the skin. (A) PrPd was detected with the anti-PrP specific antibody 6H4 in the draining inguinal LN five weeks post scrapie infection. (B) Paraffin embedded tissue (PET) blot analysis of adjacent sections confirms PrPd accumulations to be proteinase K resistant PrPSc. (C) Enlarged image of PrPd labeling from boxed area of (A). (D) shows location of FDCs via immostaining with the anti-CD21/CD35 specific antibody. PrPSc is accumulating on FDCs in the LNs.
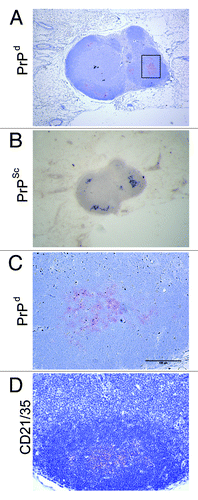
Figure 3. Depletion of DCs and macrophages in the spleen of the CD11c-DTR mouse. (B and D) CD11c+ DCs are partially depleted in this transgenic mouse line through the injection of Diphtheria toxin. CD169+ macrophages in the spleen are also depleted through their low level expression of CD11c. (A and C) Normal expression of CD11c and CD169 in control mice. (C and D) are higher magnification images of (A and B).
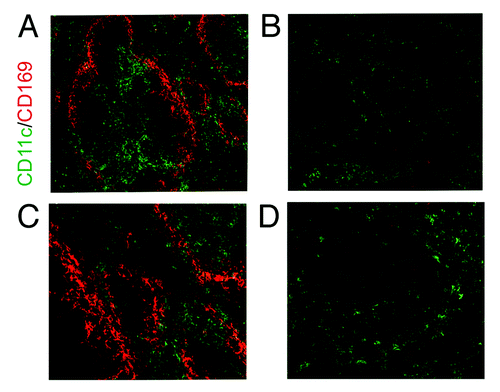
Figure 4. Detection of LCs and langerin+ dermal DCs in the mouse ear following immunofluorescent labeling of epidermal and dermal sheets. (A) LCs form a dense network of cells within the epidermis. (B) Langerin+ dermal DCs are not abundant within the dermis as the LCs in the epidermis. A small number of these cells may also be migrating LCs.
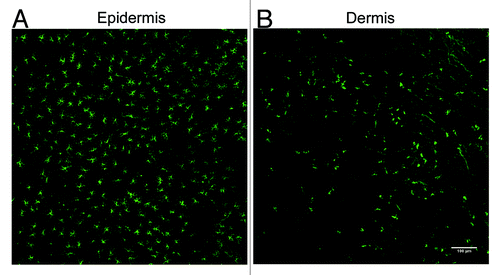
Figure 6. Schematic representation of the role of subcapsullar sinus macrophages in the transport of immune complexes to FDC in the LN. (1) Subcapsullar sinus macrophages capture lymph borne immune complexes in the subcapsullar sinus which they transcytose intact across their surfaces to underlying follicular B cells. (2) Non-cognate B cells acquire the immune complexes via their complement receptors and (3) deliver them to FDCs. (4) Cognate (antigen-specific) B cells, in contrast, acquire antigen-containing immune complexes via their B-cell receptors, become activated and (5) migrate to the boundary of the T-cell zone.
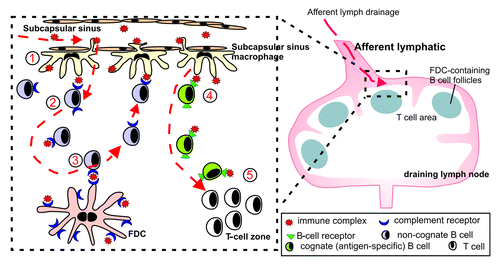
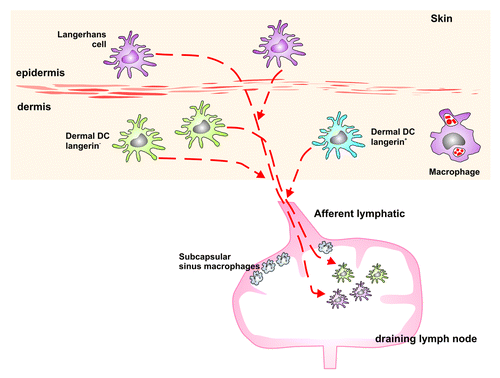
Figure 5. Schematic representation of the MNPs in the skin of the mouse. LCs can be found in the epidermis. The dermis is situated below the epidermis and comprises langerin+ as well as langerin- DCs and various macrophage populations. All these cell types migrate from the skin to the draining LN, and therefore may play a potential role in the transport of the TSE agent from the skin to the draining LN, where PrPSc accumulates following infection via the skin.