Figures & data
Figure 1. Schematic drawing of the hypothesized flow of the progressive schizophrenia disease process. A yet unidentified dysfunctionality progresses through a process that does not involve massive neuronal cell death but reflect an unidentified mechanism of permanent neuronal silencing, eventually confined to a selected neuronal population. While progressing, the lesion leads intermittently to acute symptoms.
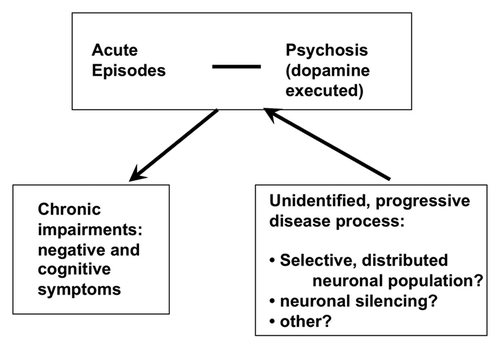
Figure 2. Laser scanning confocal microscopy of DISC1 aggregates in neuroblastoma cells. A. Mouse neuroblastoma cells (CAD cells) permanently transfected with monomeric red fluorescent protein (mRFP) transiently transfected with untagged, full length DISC1, stained with α-DISC1 mAB 14F2Citation69 and a secondary FITC-labeled antibody. Bar 10 μm. B. Human SHSY5Y cells permanently transfected with green fluorescent protein fused to human DISC1 (598–854), incubated with recombinant human DISC1 (598–854) expressed and purified from E. coli and labeled with Dylight® (red) as described by Ottis et al.Citation69 Bar 10 μm C. Human SHSY5Y cells permanently transfected with green fluorescent protein fused to human DISC1 (598–854), and incubated with synthetic α-synuclein labeled with Dylight® (red) as described by Ottis et al.Citation69 Bar 10 μm.
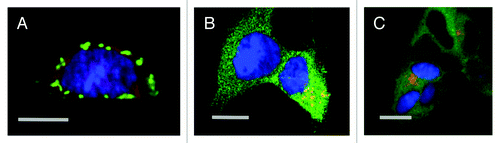
Figure 3. Schematic drawing of the size continuum of protein aggregates in chronic brain diseases. The largest aggregates visible without specific immunostaining are the extracellular Aβ and prion plaques, followed by intracellular Lewy bodies. Polyglutamine proteins are visible only after specific immunostaining. At a submicroscopic level at the bottom of this inverted pyramid are DISC1 aggregates detectable only after biochemical purification.
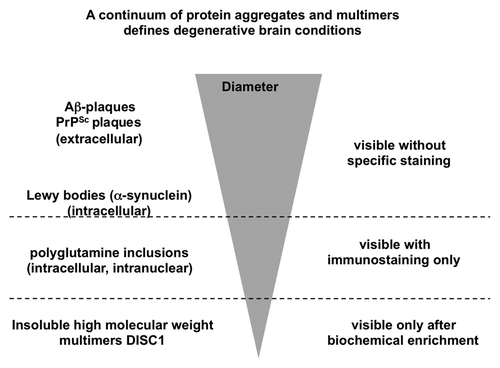
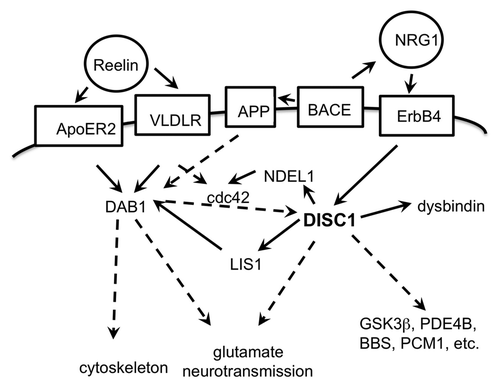
Figure 4. Schematic drawing of interactions of the DISC1 pathway. Extracellular proteins in circles, membrane proteins in boxes; direct functional connections in solid arrows, indirect functional connections (over several or unknown steps) in broken line arrows. The proteins depicted are an incomplete selection.