Figures & data
Figure 1. (A) western blot analysis of recombinant ovine PrP (rPrP), derived from bacterial inclusion bodies, with regard to proteinase sensitivity. A panel of four mAbs was used to map the N- and C-terminal ends (Anti-His and F99 respectively), the central (P4) and globular (BAR 224) domains. As shown in lanes 1, 3, 9 and 11, which were not treated with proteinase K, rPrP was detected by all mAbs with prominent dimeric (gray arrowhead) and monomeric (black arrowhead) forms. In lanes 2, 4, 10 and 12, samples were treated with proteinase K (5 µg/ml) at 22°C for 30 sec, which resulted in complete loss of signal from all mAbs except P4 which revealed a distinct triplet of bands (*), in the range of 11–14 kDa. In lanes 5, 6, 7 and 8 Proteinase K digestion continued for 1, 2, 5 and 10 min respectively. A rapid decline of signal was evident with no signal left after 10 min of Proteinase K treatment. (B) Schematic representation of rPrP with binding-sites for mAbs. Numbers refer to mAb epitopes in ovine PrP.
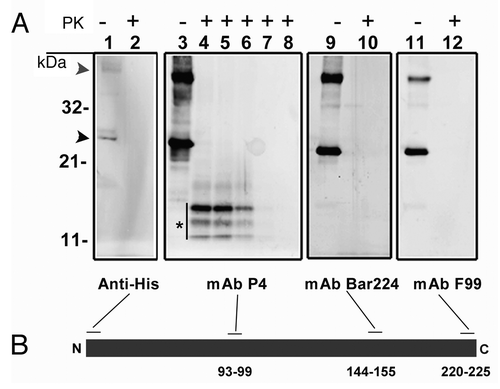
Table 1. Immunohistochemical labeling for rPrP in intestinal compartments at different time points
Figure 2. Light microscopical studies of rPrP immunohistochemical labeling (red staining) in intestinal loops of lambs 10, 30 and 60 min after inoculation. (A) rPrP labeling was detected in the lumen in close association with the apex of villous AE (07/F103 10 min BAR224), (B) and FAE of the domed PP follicle (07/F262 30 min F99). (C) rPrP labeling was observed in the lamina propria of the villi and was frequently present in the lacteals of the villi (09/F607 30 min BAR224). (D) A lower magnification shows rPrP labeled material in the villi lacteals, lamina propria, T-cell area and the submucosal lymphatics (arrow) between the PP follicles (09/607 30 min BAR 224). (E) rPrP labeling in the lumen in close contact with the FAE, and in cells in the submucosal lymphatic area (insert, magnification rPrP-positive cells) (07/F102 60 min BAR 224). (F) rPrP-labeling was also observed in intestinal tissues without PPs (07/F607 30 min BAR 224). Note that rPrP labeling was never observed in the FAE, dome or PP follicles (B, D and E). Scale bars, 50 µm, (ae) AE, (fae) FAE, (lp) lamina propria, (l) lacteal, (d) dome, (f) follicle, (t) T-cell area, (s) submucosal lymphatics, (mm) muscularis mucosa, (me) muscularis externa.
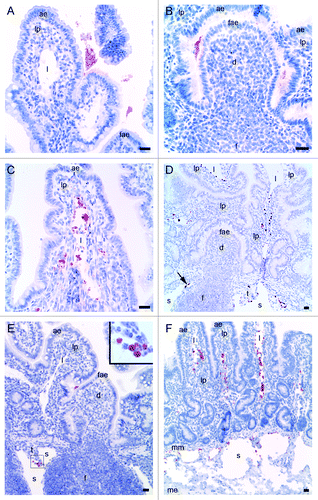
Figure 3. Immunohistochemical labeling with the PrP-antibody BAR224 showed that the inoculated rPrP was present both in cells (A) and cell-free (B) in the submucosal lymphatics. (A) The rPrP was mainly in mononuclear cells (07/F103 30 min BAR224) but also in a few polymorphonuclear cells (A, inset. 09/F607 30 min BAR224). (B) The rPrP was present in cell-free material in submucosal lymphatics (07/F289 30 min BAR224). Scale bars, 10 µm.
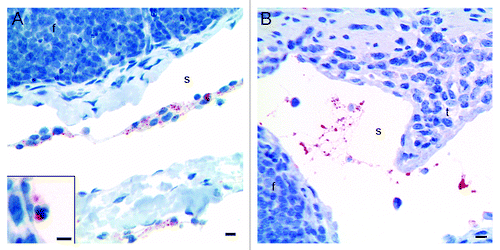
Figure 4. (A) Electron microscopy on the preparation containing rPrP demonstrated circular membrane profiles of unconfirmed origins in addition to electron dense amorphous material. (B and C) After inoculation into intestinal loops, electron dense amorphous material and circular structures were observed non-cell-associated in the lacteals (arrows) (B) and in the cytoplasm of a mononuclear cell (delineated) (C). Immunogold labeling showed rPrP labeling of inconspicuous electron dense amorphous aggregates (arrows) and on membranes of circular profiles in a lacteal (D) and in the cytoplasm of a mononuclear cell (delineated) (E). Scale bars, 1 µm. (l) lacteal, (lp) lamina propria, (e) endothelial cell, (c) cytoplasm, (n) nucleus.
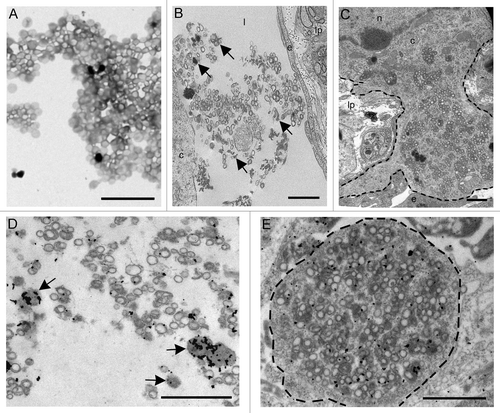
Figure 5. Immunohistochemical labeling for rPrP and Texas red fluorescence were co-localized. (A) rPrP labeling (red color) was observed in lamina propria and lacteals of a villus (09/F607 30 min BAR224). (B) The same section as shown in (A) viewed with a fluorescence microscope demonstrated that Texas red fluorescence co-localized with the immunohistochemical rPrP-labeling (arrows in both panels) (Texas red immunofluorescence filter 594). (C) immunoperoxidase labeling for rPrP without Texas red labeling in lamina propria of a villus (09/F610 30 min R145). (D) The same section as in (C) demonstrating rPrP without Texas red labeling is negative when viewed with fluorescence filter 594. Scale bar, 10 µm. (ae) AE, (lp) lamina propria, (l) lacteal, the basal lamina between AE and lamina propria is delineated.
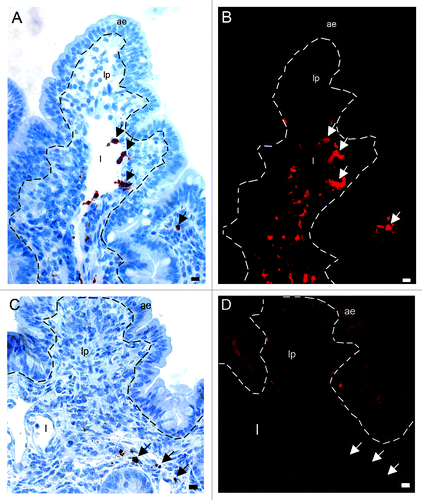
Table 2. Primary antibodies used to characterize cells in the present study
Figure 6. In situ co-localization studies of mucosal cell subsets and rPrP. (A) Multicolor immunofluorescence labeling for CD11c (blue), CD205 (green) and Texas red-labeled rPrP (red) demonstrated that rPrP was not present in double labeled CD11c+/CD205+ cells (light blue)(07/F103 30 min). Scale bar, 20 µm. A few CD205 single-labeled cells showed rPrP-labeling (yellow) in the T-cell area (arrows). (B) Despite the presence of many CD11c single labeled cells (green) in lamina propria and the dome area, these cells were not associated with rPrP (red) in any of the intestinal compartments studied. rPrP was not observed in the FAE, dome or PP follicle. (blue, hoechst nuclear staining) (09/F607 30 min). Scale bar, 50 µm. (C) Villus. The specific DC marker CD209 (green) did not show co-localization with rPrP(red) (07/F103 30 min). Scale bar, 5 µm. (D-F) Villus. Paired immunofluorescence. CD205+ cells (E, green) were found to contain rPrP (F, red). (D) The merging of panels E and F demonstrated the co-localization (yellow) of rPrP with CD205+ cells (09/F607 30 min). Scale bar, 10 µm. (G-I) T-cell area. Paired immunofluorescence. CD205+ cells (H, green) and rPrP (I, red). (G) The merging of panels H and I demonstrated the co-localization (yellow) of rPrP in a few CD205+ cells in the T-cell area (arrows) and submucosal lymphatics (arrowhead) (07/F103 30 min). Scale bar, 10 µm. (J-L) Villus. Paired immunofluorescence. MHCII+ cells (K, green) and rPrP (L, red). (J) The merging of panels K and L demonstrated rPrP in MHCII+ cells (yellow, arrows) (09/F607 30 min). Scale bar 5 µm. (ae) AE, (lp) lamina propria, (l) lacteal, (d) dome, (f) follicle, (t) T-cell area, (s) submucosal lymphatics, dashed lines indicate the basal lamina between the AE and the lamina propria.
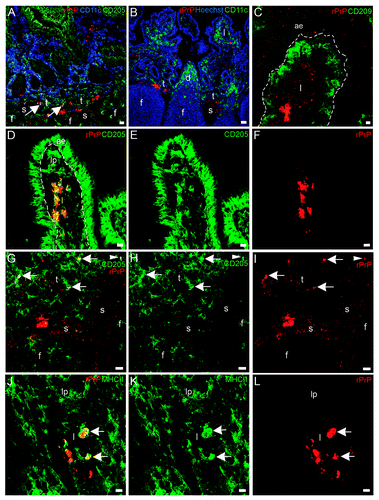
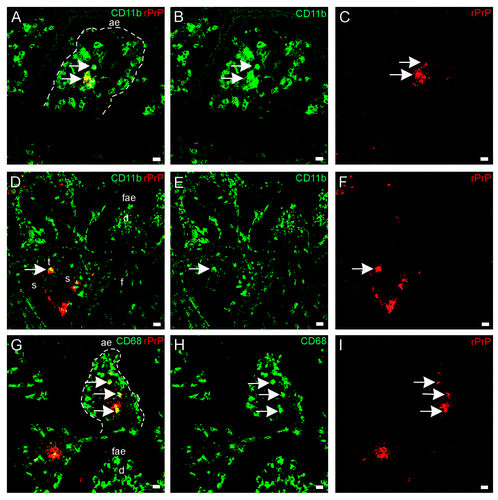
Figure 7. In situ co-localization studies of mucosal cell subsets and rPrP. (A-F) Paired immunofluorescence. CD11b+ cells (B and E, green) were found to contain rPrP (C and F, red). (A) The merging of panels B and C demonstrated the presence of rPrP in CD11b+ cells in the villus (yellow, arrows); and (D) the merging of panels E and F demonstrated co-localization in a T-cell area (arrow). Note that the FAE, dome and follicle are rPrP-negative (A-C 07/F103 30 min and D-F 09/F607 30 min). Scale bars, 10 µm (A-C) and 20 µm (D-F). (G-I) Lamina propria and dome. Paired immunofluorescence. CD68+ cells (H, green) and rPrP (I, red). (G) The merging of panels H and I demonstrates co-localization (yellow, arrows) in lamina propria, note rPrP positive material in the lumen covering FAE, but no rPrP in FAE or in the dome (09/F607 30 min). The basal lamina between the AE and the lamina propria is delineated. Scale bar, 10 µm. (ae) AE (fae) FAE, (d) dome, (f) follicle, (t) T-cell area, (s) submucosal lymphatics.