Figures & data
Figure 1 (A) Schemes of wild-type mouse PrP and Dpl. Mouse PrP is first translated as a precursor protein consisting of 254 amino acids. The N-terminal 22 and C-terminal 23 hydrophobic amino acids are removed as a signal peptide and a GPI-anchor signal sequence, respectively. The N-terminal half of PrPC is highly flexible and lacks identifiable secondary structure. The octapeptide repeat (OR) region, comprising five copies of a P(H/Q)GGG(G)WGQ octapeptide sequence, is located in the N-terminal domain. The OR region is thought to mediate anti-oxidative activity by binding to Cu2+ via histidine residues. However, the exact function of this region remains to be elucidated. The C-terminal half of PrPC forms a globular structure with three α-helices (α1–3) and two short anti-parallel β-strands (β1, β2). The second and third helices are linked by a disulfide bond (-S-S-). The precursor protein of Dpl consists of 179 amino acids. The N-terminal 25 and C-terminal 22 hydrophobic residues may be removed as signal peptide and GPI-anchor signals, respectively. Dpl is a structural homologue of the C-terminal globular domain of PrPC, sharing ∼23% identical amino acids and is composed of three α-helices (α1–3) and two short anti-parallel β-strands (β1, β2). Two disulfide bonds (-S-S-) are formed. However, Dpl lacks the corresponding N-terminal part of PrPC. (B) Structural schemes of PrPs with deletion of various regions and PrPN-Dpl, the fusion protein composed of the N-terminal region of PrP with Dpl, with their cis- and trans-protective activity against Dpl or toxic PrPs are shown. a: Construct 7 is itself non-toxic. However, it has different affects on neurotoxic Constructs 2 and 6: It enhances the toxicity of Construct 6 but diminishes that of Construct 2. NA: data are not available.
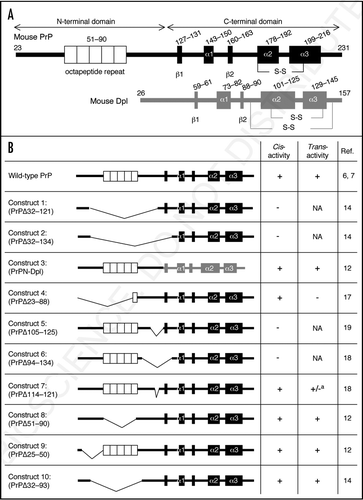
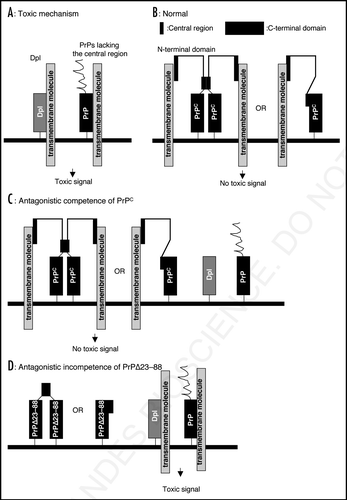
Figure 2 A possible mechanism for the antagonistic interaction of PrPC and Dpl or the toxic PrPs. (A) Dpl binds to a putative transmembrane molecule, producing a toxic signal. Toxic PrPs with deletion of the central region, such as Constructs 1, 2, 5 and 6, bind to the transmembrane molecule via the Dpl-homologous C-terminal area in the same way as Dpl, eliciting a similar toxic signal. (B) Under normal conditions, wild-type PrPC binds to the trans-membrane molecule via the N-terminal region but not its C-terminal region because it forms either a homo-dimer linked via the central region or a monomer with the central region interacting with part of the C-terminal domain. The N-terminal region acquires binding affinity to the molecule only when the central region is intact. However, this type of interaction produces no toxic signal. (C) PrPs with part of the N-terminal region and with the central region both intact, such as trans- protective PrPs, have a higher affinity for the transmembrane molecule than Dpl or the toxic PrPs, resulting in trans-protection against Dpl and the toxic PrPs. (D) Construct 4 (PrPΔ23–88) still has potential to form a homo-dimer due to the residual central region or a monomer with the residual central region masking part of the C-terminal region, similarly to wild-type PrPC. Therefore, Construct 4 cannot form a complex with the transmembrane molecule via the C-terminal region, generating no toxic signal. In addition, by lacking part of the N-terminal domain, Construct 4 has no affinity for the transmembrane molecule, losing trans-protective activity against Dpl.