Figures & data
Figure 1 Isolated amyloid fibrils composed of Aα chain fragment of fibrinogen (a) stained with Congo red and visualized by light microscopy and (b) between crossed polars, showing characteristic apple-green birefringence. Figure adapted from reference Citation83.
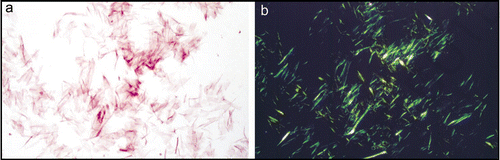
Figure 2 Synthetic amyloid fibrils made from Aβ peptide (A) electron micrograph showing long, straight, unbranching fibrils. (B) X-ray fiber diffraction pattern from partially aligned amyloid fibrils showing the characteristic “cross-β” diffraction pattern. (C) The structure of the Aβ amyloid fibril interpreted from ssNMR data,Citation67 showing the top view of the fiber (i and ii) with side chains (i), showing the importance of side chain packing with in the fiber and as a cartoon (ii). The side view (iii) revealing the β-strands running perpendicular to the fiber axis.
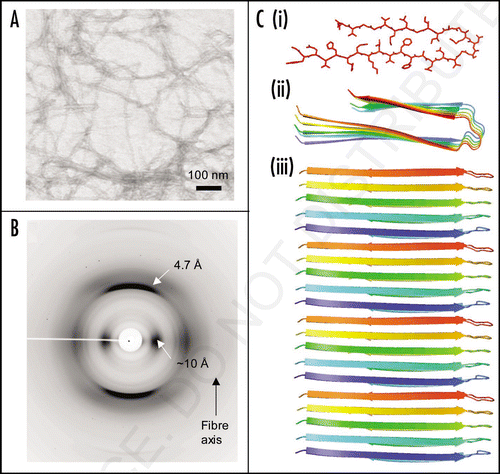
Figure 3 Models of mature protein fibrils based on Small-Angle X-ray scattering solution data. (A) Human alpha-synuclein fibrils and (B) human insulin fibrils.Citation69 The results suggest that insulin fibrils (B) are formed of three intertwining protofibrils, whereas a-synuclein fibril (A) consist of only one protofibril. Each protofibril is assumed to consist of two intertwining protofilaments. Four and three repeating units are shown for alpha-synuclein and insulin respectively.
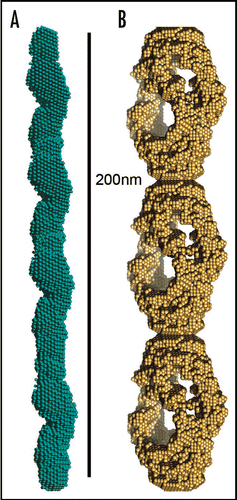
Figure 4 Amyloid-like fibers for bionanotechnology. (A) Nanowires based on the N-terminal region of the yeast prion, Sup35. Nanogold was covalently linked to the engineered cysteine residues in the protein and conjugate colloidal gold and silver particles were associated along the fibers to form wires,Citation80 (B) assembly of diphenylalanine to form nanotubes that can be filled with silver to make nanowires.Citation82
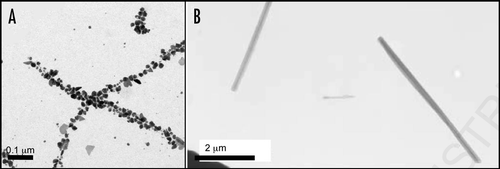
Table 1 Some amyloidoses and their respective precursors and amyloid-ogenic proteins