Figures & data
Figure 1 The role of Hsp104, Hsp70 and Hsp40 in Sup35 prionogenesis. (A) Sup35 prions assemble after a lag phase during which a dynamic ensemble of monomeric and molten oligomeric species form. The intermolecular contacts that nucleate prion assembly are likely established within amyloidogenic oligomers. Sup35 fibers are held together by an alternating sequence of Head-to-Head (pink) or Tail-to-Tail (dark blue) intermolecular contacts that are separated by a central core region (light blue) comprised of intramolecular contacts. Once formed, fibers stimulate their own assembly by recruiting and converting monomers at their ends. Various steps are promoted or antagonized by Hsp104, Hsp70, Hsp40 and the NEFs Fes1 and Sse1 as indicated. At high concentrations, Hsp70 and Hsp40 can bind Sup35 prions and occlude prion recognition elements. The C-terminal domain of Sup35 is not depicted for clarity. (B) Incorporation of Ssa1 and Ssb1 into Sup35 prions has distinct effects on subsequent Hsp104 remodeling. Ssa1 incorporation protects fibers against remodeling by Hsp104 to non-infectious conformations, perhaps by maintaining active fiber ends. Ssb1 incorporation promotes the remodeling of fibers to non-infectious conformations, perhaps by promoting inactivation of fiber ends. The majority of Ssb1 is associated with the ribosome.Citation29 However, a considerable fraction is found throughout the cytoplasm,Citation29 which is likely to be able to interact with Sup35 prions.
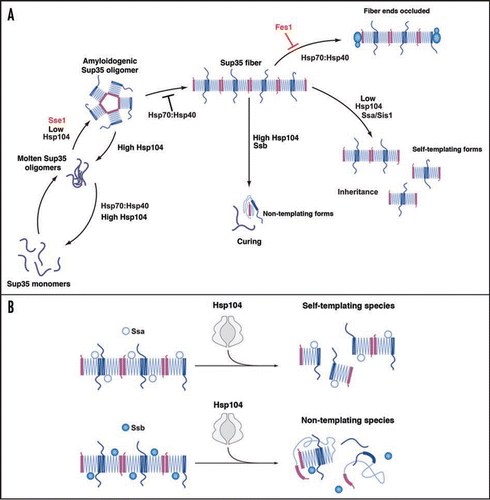
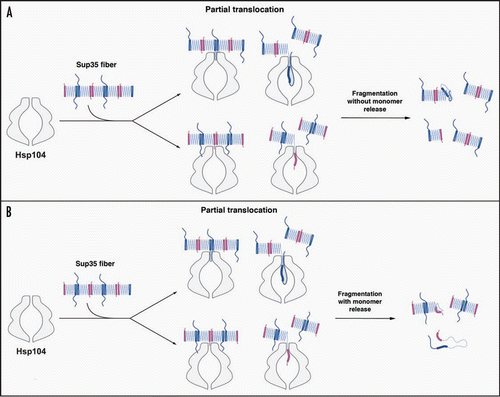
Figure 2 Possible modes of Sup35 prion fragmentation by Hsp104. (A, B) Partial translocation might disrupt Head-to-Head (pink) or Tail-to-Tail (blue) contacts without (A) or with (B) monomer release. Partial translocation might occasionally leave some fiber ends inactivated. The C-terminal domain of Sup35 is not depicted for clarity.