Figures & data
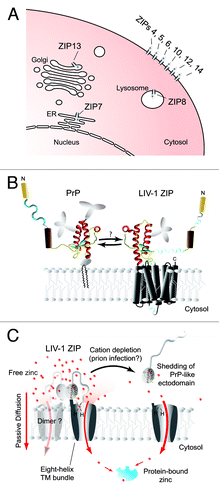
Figure 1. Localization, function and possible interaction of LZTs with the prion protein. (A) Cartoon depicting the reported predominant cellular localization of LIV-1 ZIP transporter (LZT) family members. Whereas more distantly related LZT metal ion transporters may preferentially associate with a variety of intracellular organelles, members of the phylogenetic subbranch of LZTs comprising ZIPs 5, 6 and 10 are predominantly observed at the plasma membrane. (B) Model of prion protein next to an LZT, depicted with its predicted PrP-like ectodomain. The interface that mediates the interaction between PrP and LZTs is as yet undefined. (C) Model of LZT-mediated cellular zinc import through passive diffusion and shedding of the PrP-like ectodomain in response to extracellular metal starvation. It should be noted that a similar endoproteolysis of a subset of LZTs can be observed in prion-infected mouse brains (unpublished dataCitation20). Panels B and C were modified from a Progress in Neurobiology articleCitation3 with permission from Elsevier.