Figures & data
Figure 1. PrP surface expression. Shown are flow cytometic analyses plots of neuronal cells labeled with antibody 6H4. A secondary antibody, GAM-FITC, was used to visualize the cells. The plots to the left shows non-specific GAM-FITC binding following incubation with an irrelevant isotype control IgG antibody. The plot to the right depicts cell fluorescence following incubation with the PrP-specific antibody 6H4 and the FITC-conjugated GAM. The % shows the percent of cells which have shifted, indicating fluorescence. Media control cell autofluorescence (no antibody added) resulted in 1.05% of cells indicating fluorescence (data not shown).
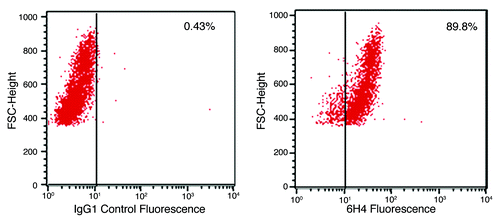
Figure 2. Peptide array pseudo-image. Shown is a pseudo-image of a peptide array block combining the prion protein stimulation data. Transformed data for PrP 106-126 is compared with scramble control peptide data, and a significance value is determined based on a significant increase or decrease in phosphorylation between the two. This process is also performed for 6H4 antibody stimulated and isotype control antibody. The relative intensity of each spot indicates the level of significance of the phosphorylation of that spot. The left side of each spot is the response to PrP 106-126 stimulation. The right side of each spot indicates 6H4 stimulation response. Grey spots represent inconsistent peptides between array peptide replicates as determine by Χ2 test
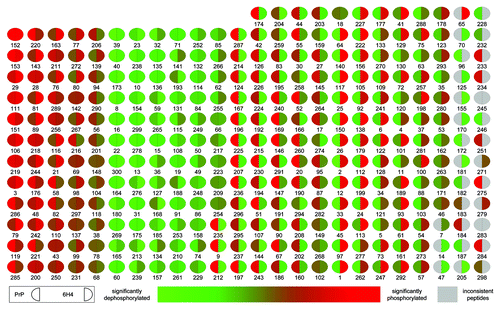
Figure 3. Phosphorylation Heat Map and Clustering. (A) The background-corrected raw data collected from the peptide arrays were VSN-transformed, and a heatmap/clustering of the data was produced using the Complete Linkage + Euclidian Distance method. The lines at the top of the heatmap indicate the relative similarity between the stimulants indicated at the bottom of the heatmap. The shorter the lines, the more similar the two connected stimulants. The lines on the left side of the heatmap indicate the relative similarity in signal between the 300 individual peptides on the array. The colored lines indicate the relative degree of phosphorylation of each peptide from strongly phosphorylated (red) to non-phosphorylated (green) as indicated by a Z-score. (B) Shown here is the 3D Principal Component Analysis of the five treatments. Relative distance on the three axes indicates level of similarity or difference among the treatments. PrP refers to PrP 106-126 peptide. Scram refers to scramble control peptide. 6H4 refers to the PrP-specific antibody. Iso refers to the IgG1 isotype control antibody. Media refers to media control.
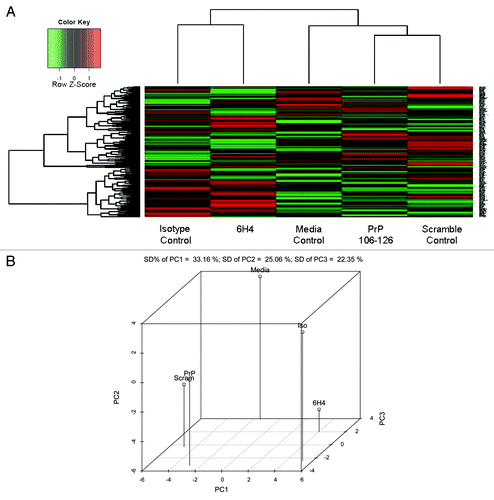
Figure 4. Stimulation comparison of peptide phosphorylation. Peptides displaying differential phosphorylation following the two stimulations are compared. The majority of the significantly differentially phosphorylated peptides are unique to one stimulant or the other. A minority of the peptides are common to both PrP 106-126 and 6H4. Common Direction of Phosphorylation refers to peptides displaying either increased or decreased phosphorylation following both stimulations. Opposite Direction of Phosphorylation refers to one stimulation resulting in an increased phosphorylation, while the other stimulation results in a decrease.
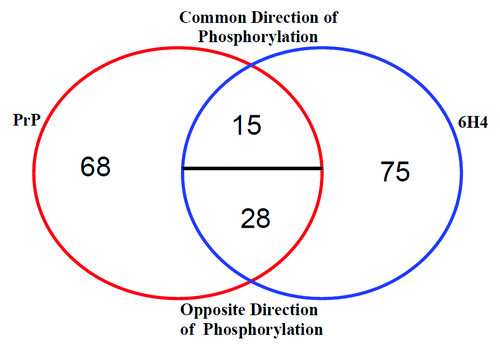
Table 1. Innate DB generated pathway list
Figure 5. Signaling pathways linked to PrPC stimulation. Shown is a selection of proteins implicated by peptide array data that is organized into interconnected pathways. Yellow indicates unique PrP 106-126-related phosphorylation. Orange indicates unique 6H4-related phosphorylation. Red indicates that both stimulants affect the peptide in the same direction of phosphorylation. Green indicates that both stimulants affect the peptide in opposite directions. Clear indicates insignificant signal or that the peptide is not present on the array.
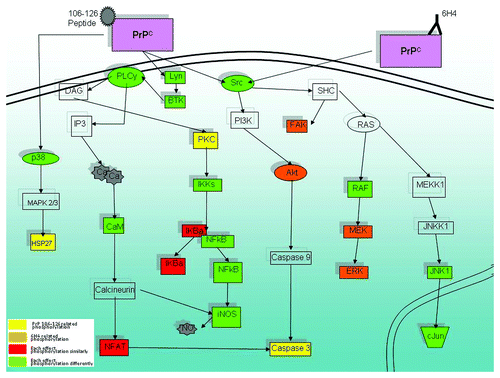
Table 2. Phosphorylation of select signaling molecules indicated by peptide array and phosphospecific antibody array
Figure 6. Cell viability. Stimulated human neuronal cells were assayed for viability using trypan blue dye exclusion and a hemocytometer. Camptothecin refers to the inducer of apoptosis. PrP 106-126 refers to the prion peptide fragment. 6H4 refers to the 6H4 antibody. PrP 106-126 + VEGF refers to PrP 106-126 peptide plus the addition of recombinant VEGF. 6H4 + VEGF refers to 6H4 antibody plus recombinant VEGF. Each stimulated sample was compared with its respective control sample to keep analyses as similar to peptide array analyses as possible. Camptothecin and VEGF counts were compared with unstimulated control counts. 6H4 counts were compared with IgG1 isotype control counts. 6H4 + VEGF counts were compared with 6H4-stimulated counts. PrP 106-126 counts were compared with scramble peptide control counts. PrP 106-126 + VEGF counts were compared with PrP 106–126-stimulated counts. Assays were performed in triplicate with fold change and standard error presented. *Indicates a statistical confidence of p ≤ 0.05.
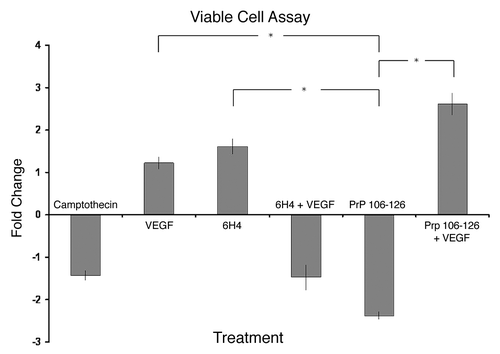
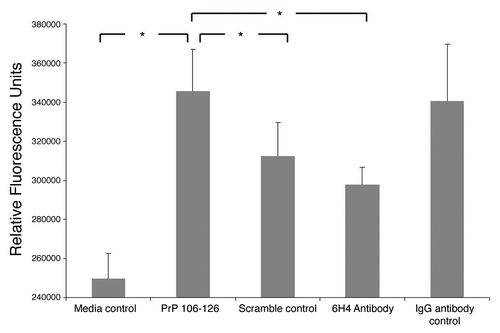
Figure 7. Intracellular calcium. Stimulated human neuronal cells were assayed for intracellular calcium as measured by fluorescence. The assay was performed in triplicate. The means of the replicates are presented along with standard error of the means. Analysis of variance indicated a p-value of 0.032 between groups. The greater the fluorescence signal, the more intracellular calcium. Pairs which showed a statistically significant difference in signal of at least p ≤ 0.05 are indicated.