Figures & data
Figure 1. Schematic presentation of PrP trafficking in infected cells and upon OHT treatment. In infected cells PrPC and PrPSc interact at the plasma membrane in cholesterol-rich lipid domains called lipid rafts. Upon internalization, both PrPC and PrPSc can recycle via the endosomal recycling compartment (ERC) or can be routed for degradation in lysosomes. Subcellular cholesterol distribution influences PrPSc trafficking in the endocytic pathway. In untreated, infected cells, the majority of PrP recycles through the cholesterol-rich ERC, supporting conversion of PrPC to PrPSc. Treatment with 4- hydroxytamoxifen (OHT) induces cholesterol accumulation in enlarged late endosomes (LE). PrPSc production and degradation defines the cellular load of infectious prions. We propose that 4-hydroxytamoxifen-induced changes in PrPSc trafficking favor PrPSc degradation. EE, early endosomes; LYS, lysosomes. (Adapted with permission from Marzo et al., 2013).Citation14
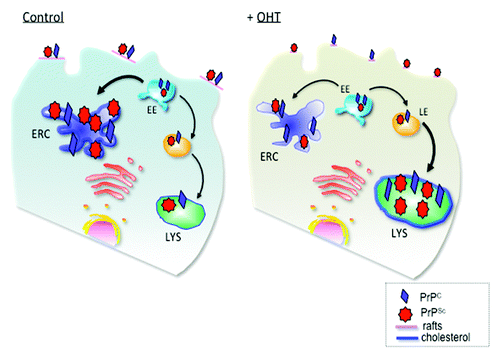
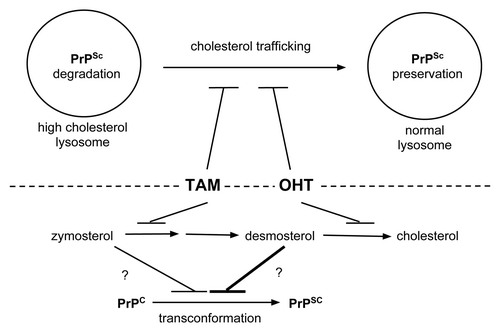
Figure 2. Possible mechanisms of prion clearance by tamoxifen and OHT. Above dashed line: Tamoxifen and OHT may impair trafficking of cholesterol out of lysosomes. The high cholesterol environment favors PrPSc degradation and/or impairs PrPSc transconformation from PrPC substrate molecules (left lysosome). In contrast, without drug treatment normal cholesterol trafficking supports an environment that prevents PrPSc degradation and/or favors PrPSc formation (right lysosome). Below dashed line: Tamoxifen and OHT inhibit cholesterol biosynthesis at different steps. While tamoxifen treatment leads to zymosterol accumulation, OHT leads to desmosterol accumulation.Citation20 Desmosterol may be a more potent inhibitor of PrPSc formation or promoter of its degradation than zymosterol as indicated by the differently weighted lines impacting on PrPSc formation.