Figures & data
Figure 1. Breeding schemes and experimental groups. Three experimental groups were designed in order to investigate a possible sexual and parental transmission of prion disease. (A) Male and female hamsters were i.p. injected with 263K prions. Animals were sacrificed at stage 4 of the disease as previously reportedCitation46 and sexual organs were collected to assess PrPSc content by WB and PMCA. (B) Breeding pairs using different combinations of infected and un-infected males and females were set in order to assess a putative prion transmission by sexual contact. (C) Pups generated from breeding in (B) were kept and observed for appearance of prion disease.
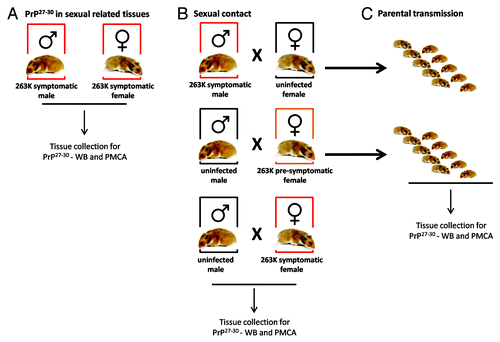
Table 1. Summary of groups, conditions and results obtained
Figure 2. Survival curves of intra-peritoneally infected 263K male and female Syrian hamsters. ~40 d old male (n = 10) and female (n = 11) hamsters were intra-peritoneally infected with 263K prions as described in Materials and Methods. Animals were sacrificed at advanced stage of clinical disease. Numbers in parenthesis note average incubation periods ± standard error. Survival curves were significantly different (P value = 0.0007).
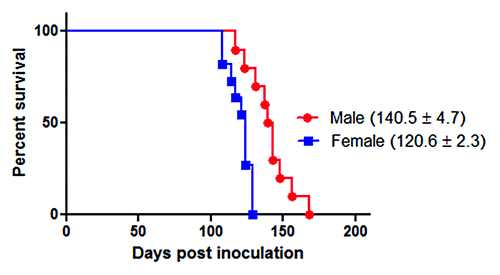
Figure 3. Western blotting and PMCA assessment of PrPSc in sexual organs. Male and female 263K infected hamsters were sacrificed at the clinical stage of the disease and sexual organs (ovaries, uteruses and testes) were collected. (A) WB analyses of PK treated tissue homogenates from selected animals. (B) PMCA analyses of same organs showed in (A) plus ocular and intestinal tissue homogenates. For space constrains, the results of 2 representative animals of the 10 studied are shown (1 and 2). (C) PMCA of brain dilutions from a sarkosyl cleared brain homogenate used as a positive control of in vitro amplification. (D) Un-seeded PMCA reaction used as a negative control. All PMCA generated samples (B–D) were PK treated before WB. PrPC correspond to brain homogenates from healthy hamsters (no PK treated) used as a control of electrophoretic mobility. Black horizontal lines at the right of each gel represent a 26 KDa molecular weight marker. Dotted line depicts splicing of two different gels. Numbers in (B) and (D) indicate samples from different animals.
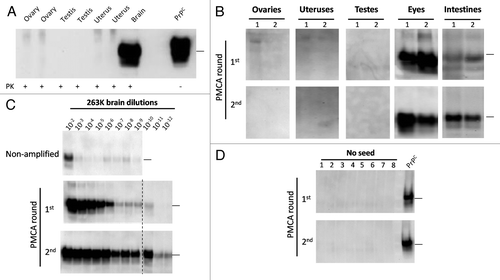
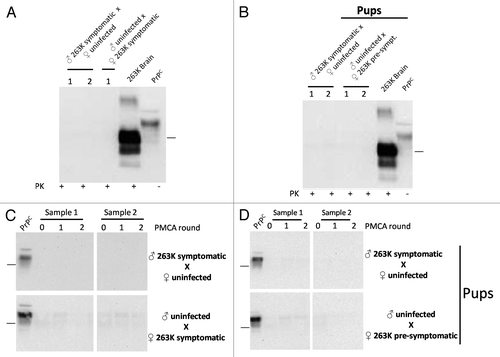
Figure 4. Biochemical assessment of PrPSc after sexual and parental prion contact. Brain homogenates from males and females having sexual contact with infected animals were tested for WB (A) and PMCA (C). The brain of the uninfected animal depicted in the breeding is the one tested for PrPCitation27-Citation30 by either WB or PMCA. Brain homogenates of pups coming from infected mothers or fathers were also tested by WB (B) and PMCA (D). All PMCA generated samples (C, D) were PK treated before WB. The samples showed in panel d correspond to the brain of either male or females born from the breeding illustrated in the respective blot. 263K brain and PrPC (PK and non-PK treated, respectively) corresponds to brain homogenates from infected and healthy hamsters used as a control of electrophoretic mobility. Black horizontal lines at the right of each gel represent a 26 KDa molecular weight marker. Numbers 1 and 2 in (A) and (B) and samples 1 and 2 in panels (C) and (D) indicate samples coming from different animals, which are representative of all animals analyzed.