Figures & data
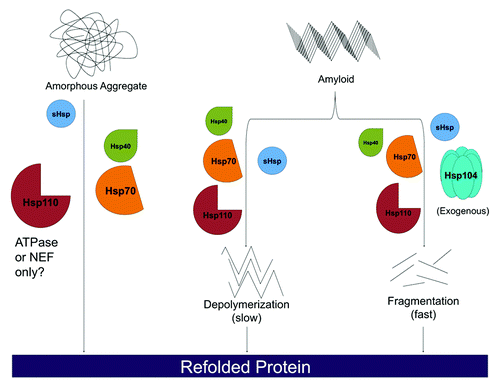
Figure 1. Hsp110, Hsp70, Hsp40 and sHsps are a disaggregation system in metazoan cells. Heat shock proteins Hsp110, Hsp70, and Hsp40 are capable of dissolving disordered aggregates. For labile aggregates, Hsp110 may only need to operate as a nucleotide exchange factor (NEF) for Hsp70, whereas for more stable aggregates it may need to serve as a NEF for Hsp70, engage substrate, and bind and hydrolyze ATP. Hsp110, Hsp70, and Hsp40 can also slowly depolymerize ordered amyloid substrates from their ends. Rapid amyloid dissolution can be achieved by supplementing Hsp110, Hsp70 and Hsp40 with exogenous Hsp104. Here, fibrils can be fragmented and monomers extracted from anywhere in the fibril (not just the ends), which leads to more rapid dissolution. sHsps can stimulate all of these protein disaggregation reactions, but are not absolutely required.