Figures & data
Figure 1 Dynamics behavior of the radii of gyration (Rg) of Aβ40 during MD simulations performed for (A and B) initial structures at both considered pHs. Notes: Plots of the radii of gyration calculated (in Angstrom) for backbone atoms (Cα, C, N) of Aβ40 at pH 8 and pH 3 are shown in the upper and lower parts of the figure, correspondingly. Colored curves correspond to the A (magenta) and B (blue) initial structures (see Methods).
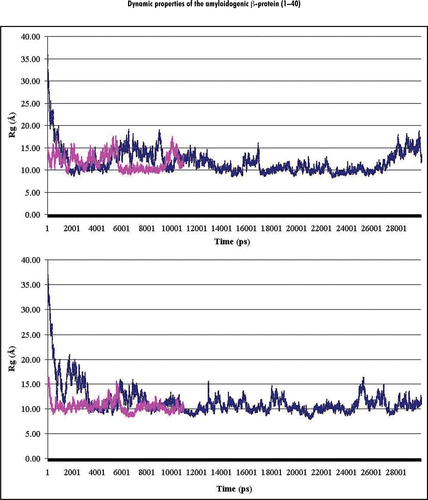
Figure 2 Percentage (%) of involvement of the amino acid residues within the Aβ40 sequence in forming the secondary structural elements (structural propensity) during the last 10 ns of MD simulations at pH 8: (A and B) present secondary structure sets determined by the analyses of the RSA8 and RSB8 structural sets, correspondingly. Notes: The secondary structure elements were depicted by different colors: α-helix (green), 310-helix (blue), β-turn (yellow) and random coil (red).
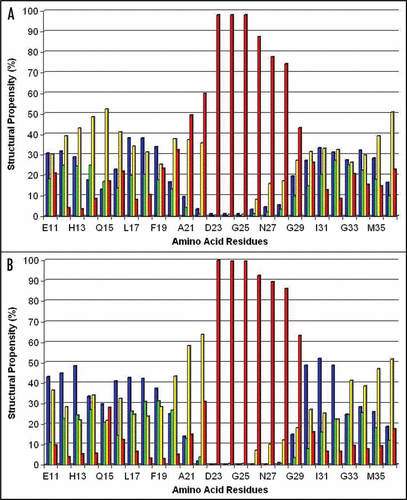
Figure 3 The trace of the Cα-atoms of the average Ω-shaped structure formed by the Ala21-Gly29 segment in the stable state at pH 8.
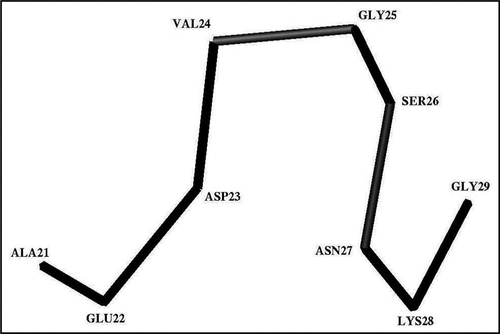
Figure 4 A typical three-dimensional structure formed by the Glu22-Gly29 segment in the stable state at pH 8. Notes: Observed H-bonds between polar groups are shown by dash-lines. CD, CG, OG and NZ note the heavy atoms of the donor-acceptor groups of the interacting residues.
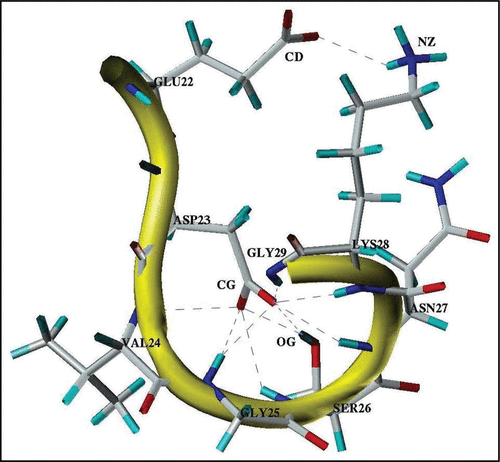
Figure 5 Schematic presentation of the major structural states of the Glu11-Val36 segment of the Aβ40 core domain considered at pH 8 and pH 3. The rectangular blocks along the sequence designate the flexible and fluctuating helix-like structural modes: Glu11-Phe20 (Helix 1′) at pH 8; Glu11-Val24 (Helix 1) at pH 3, and Ala30-Val36 (Helix 2) at both pHs. The Ala21-Gly29 residues at pH 8 form the Ω-shaped structure (comprised of about 84% of the MD generated structures). The Gly25-Gly29 residues at both pHs form a bend-shaped structure.
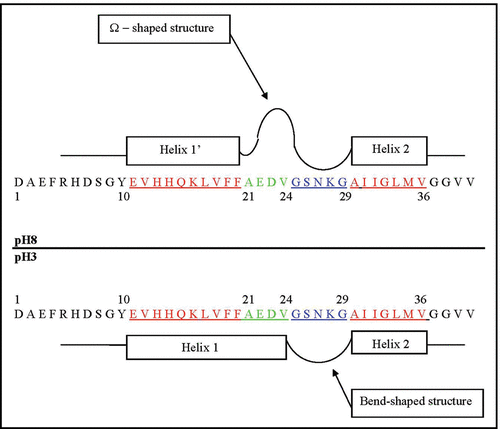
Figure 6 Helical net layout of the Glu11-Gly37 segment. Notes: The sequence is presented by the net diagram typical for two α-helices, formed by the Glu11-Val24 (Helix 1) and Ala30-Val36 (Helix 2) segments. The residues forming the continuous hydrophobic clusters are marked by dark circles. The residues forming the bend structures (Gly25-Gly29) are marked by the hexagons. The numbers present the positions of the corresponding amino acid residues in the Aβ40 sequence.
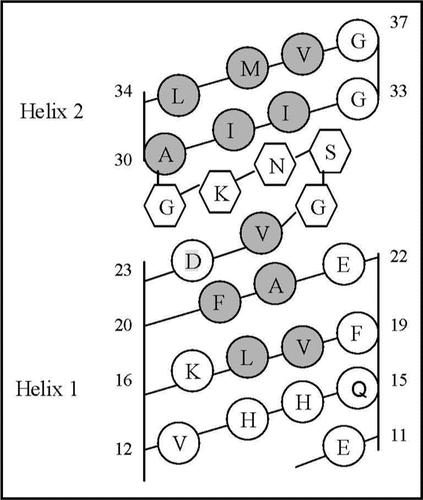
Table 1 The structural propensities (occupation percentage) of amino acid residues within Glu11-Val39 segment to be involved in the formation of secondary structure elements revealed from the RSA8 and RSA3 structural sets at the last 10 ns of the MD simulations
Table 2 The structural propensities (occupation percentage) of amino acid residues within Glu11-Val39 segment to be involved in the formation of secondary structure elements revealed from the RSB8 and RSB3 structural sets at the last 10 ns of the MD simulations RSA8 RSA3
Table 3 Percentage (%) of helical structures adopted by five tetrapeptide segments of Aβ40 at pH 8 and pH 3
Table 4 The percentage (%) of structures assigned to the most populated clusters formed by the Ala21-Gly29 segment and overall description of the structures formed by the Ala21-Val24 residues
Table 5 Percentage of the cases (%), in which the hydrogen bonds (H-bonds) between the donor-acceptor groups of the residues within the Glu22-Gly29 segment are formed