Figures & data
Figure 1 Oligomeric interfaces of β2m. The β2m hexamer is shown as a semi-transparent surface flanked by surface cut-away views of each interface. In the center panel, chains are alternately colored blue and green. This illustrates the two types of interface present. In the left and right panels, one chain from each interface is represented as a solvent accessible surface, the other as sticks. Orange and red correspond to contact residues that have moved <2 Å or >2 Å r.m.s. respectively relative to the same residues in WTapo structure, PDB code 2CLR.Citation43 For the residues that have moved >2 Å r.m.s., side chains of WTapo are also drawn (blue) with their position in hexameric β2mholo indicated by arrows.
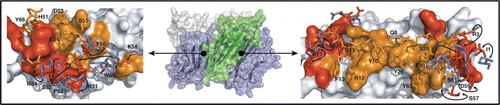
Figure 2 Cu2+ binding to β2m and PrP. (A) Cu2+-site derived from the Cu2+-bound β2m hexamer (PDB 3CIQ)Citation25 and (B) Cu2+-site derived from the HGGGW fragment of the PrP octapeptide repeat.Citation26 Both sites display coordination by an imidazole ring, two backbone nitrogens and a backbone carbonyl in a square-planar arrangement. Note that the PrP structure also contains an axial water ligand which was omitted from this figure.
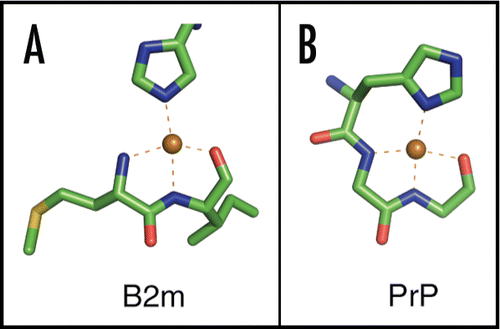
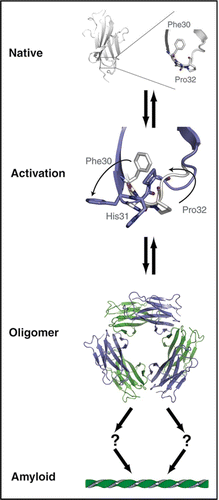
Figure 3 Unified model of β2m amyloid formation: Under physiological conditions, β2m exists as a stable, well folded monomer (upper panel, PDB 2CLRCitation43) characterized in part by a conserved cis proline at residue 32. Upon exposure to a variety of amyloigenic triggers the native structure is perturbed and amyloid formation commences. We have shown (Cu2+ or P32X mutation) or conjectured (limited proteolysis or acidic pH) that this transition involves rotation of Phe30 from the hydrophobic core toward solvent and the cis-trans isomerization of Pro32.Citation20,Citation25 We postulate that all pathways converge on a state resembling the activated monomer (M*) in which broad rearrangements occur to compensate for the cavity left by movement of Phe30. These rearrangements precede oligomerization which terminates in a hexamer.Citation10,Citation20,Citation25 The path from oligomer to mature aggregate is not known, however, as the hexamer represents a closed state, aggregation likely requires a ring-breaking event.