Figures & data
Figure 1 Representative western blot for the expression of PrPC in bovine tissues. (A) PrPC displayed three distinct migration bands corresponding to molecular weights of 35, 28 and 25 kDa. PrPC was detected in all tissues analyzed with greatest expression in CNS tissues (cerebellum, obex, spinal cord). GAPDH was used as control protein (30 kDa) and was present in all samples. (B) Cerebellum, obex and spinal cord showed the highest (p < 0.05) levels of immunoreactivity for PrPC. Thymus expressed highest levels of PrPC among non-neural tissues. Different superscripts letters indicate significant differences (p < 0.05). Ce, cerebellum; Ob, obex; SC, spinal cord; Th, thymus; In, intestine; Ne, nerve; He, heart; Sp, spleen; Lu, lung; Mu, muscle; Ki, kidney; LN, lymph node; Sk, skin; Pa, pancreas; Li, liver.
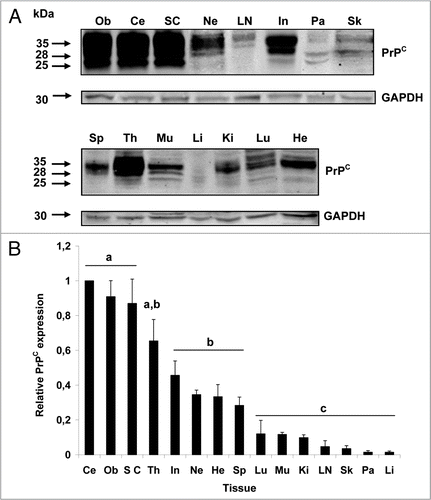
Figure 2 Expression of PrPC in bovine neural tissues. Transverse tissue section incubated with SAF-32 antibody and stained using peroxidase. (A) PrPC staining (brown) is intensely present in Purkinje cells (arrows) and cells of the molecular layer (ML) and granular layer (GL) in the cerebellum. Less immunoreactivity is observed in the white matter (WM). (B) Higher magnification shows intense staining in fibers of the ML, Purkinje cells (arrows) and neurons of the GL. (D) In the solitary tract nucleus of the obex, PrPC is associated to neuronal bodies, neuropil and neuroglia. (E) Higher magnification shows labeling of PrPC in neuronal bodies, appendixes and glial cells (arrow-heads). (G) PrPC immunoreactivity in the spinal cord is confined to the gray matter (GM) with low intensity in the white matter (WM). (H) In the sciatic nerve, PrPC staining is restricted to neural fibers associated in fascicles (F). No PrPC labeling was observed in the perineurium (P). Inserts and figures C (cerebellum) and F (obex) represent serial sections incubated with non-immune horse serum instead of SAF-32 antibody (negative control).
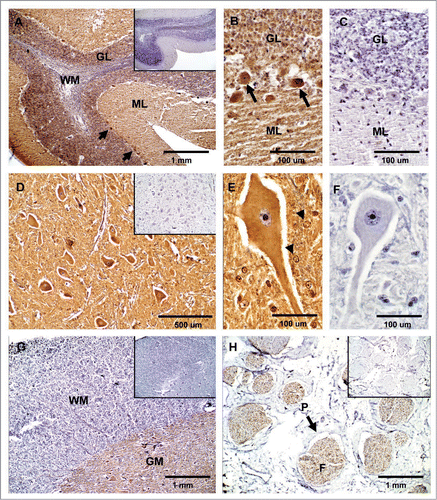
Figure 3 Expression of PrPC in bovine lymphatic tissues. (A) PrPC-specific labeling is greatest in the cortex (Cx) of the thymus and moderate in the medulla (M). (B and C) Higher magnification in the cortex area shows PrPC positive (arrows) and negative (arrow-heads) thymocytes. (D) In the spleen, staining for PrPC was observed in perilymphoid zones surrounding nodules of white pulp (WP) (RP, red pulp). (E and F) Higher magnification shows presumably PrPC positive myeloid DCs (arrow) in the spleen. (G) PrPC-specific labeling is associated with lymphoid follicles (LF) present in the cortex area of the mesenteric lymph node. (H and I) Higher magnification evidence specific staining for PrPC presumably associated with lymphocytes (arrow) surrounding the lymphocyte corona (LC) and germinal centers (GC). Inserts represent serial section incubated with non-immune horse serum instead of SAF-32 antibody (negative controls).
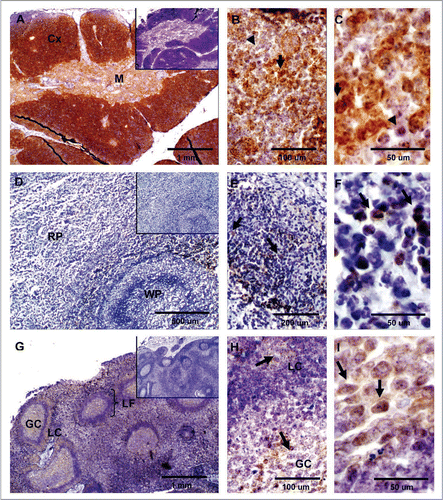
Figure 4 Expression of PrPC in bovine digestive tissues. (A) Sagittal tissue section of the ileum shows PrPC-specific labeling in lamina propia (*), muscularis (arrowhead) and myenteric plexus (arrow). (B) Higher magnification of neurons positive for PrPC in the lamina propia (arrow in B and * in A). (C) PrPC is highly expressed in parasympathetic ganglion cells forming the myenteric plexus (arrow in Fig. A and C). (D) PrPC positive staining is restricted to the endocrine pancreas in the islets of langerhans. (E) Higher magnification shows specific PrPC-positive pancreatic endocrine cells. (F) No PrPC staining was observed in the liver tissue after incubation with SAF-32 antibody. Inserts represent serial section incubated with non-immune horse serum instead of SAF-32 antibody (negative control).
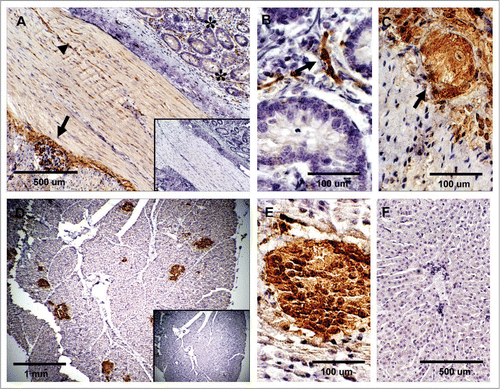
Figure 5 Expression of PrPC in bovine striated and cardiac muscle. (A) No immunoreactivity for PrPC was detected in semitendinosus striated muscle after incubation with SAF-32 antibody. (B) PrPC labeling was observed in unidentified structures outside the cardiac muscle fibers; labeling was not observed in cardiac muscle cells of the myocardium. Inserts represent serial section incubated with non-immune horse serum instead of SAF-32 antibody (negative control).
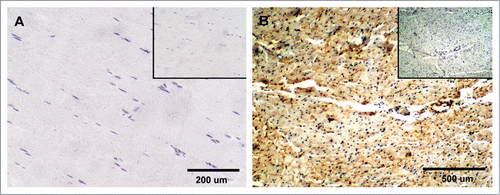
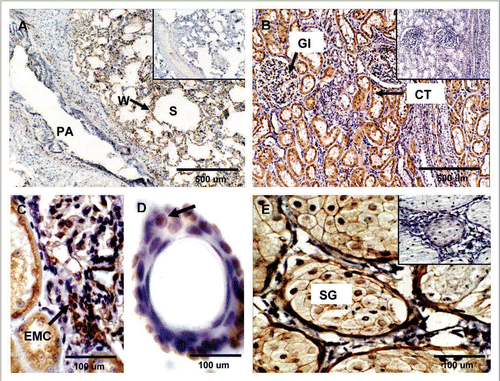
Figure 6 Expression of PrPC in bovine lung, kidney and skin. (A) PrPC-specific labeling was observed associated with the alveolar wall (W) (arrow) (Alveolar Sacs, S; Pulmonary Artery, PA). (B) In kidney, PrPC immunoreactivity is associated with glomeruli (Gl), proximal convoluted tubules (CT) and collecting ducts in the medulla. (C) Higher magnification of renal glomeruli shows strong PrPC staining in extraglomerular mesangial cells (EMC). (D) PrPC staining in the skin is associated with epidermal cells in hair follicles (arrow). (E) Staining was also present in sebaceous glands (SG) located in the dermis. Inserts represent serial section incubated with non-immune horse serum instead of SAF-32 antibody (negative control).