Figures & data
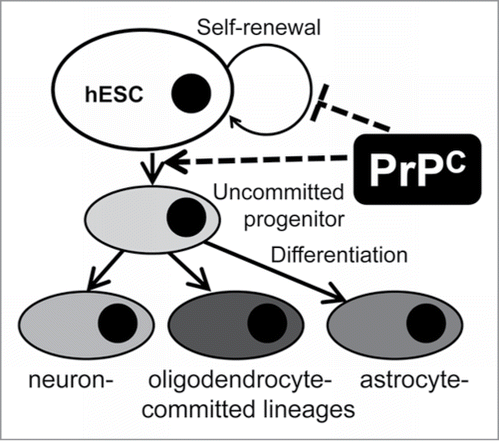
Figure 1. Suppression of PrPC delays differentiation into neuronal cells. (A) Phase-contrast images of hESCs with suppressed PrPC-expression (Sup) and in two control lines (C1 and C2) taken at 10th, 20th, 30th, and 40th day of differentiation. (B) Immunostaining for PrPC (red) and TH (green) on 40th day of differentiation. Hoechst 33342 was used for staining of nuclei (blue). Scale bar = 50 μm. (C) Quantification and statistical analyses of PrPC- and TH-positive cells on 40th day of differentiation. The data represent a mean ± SD from three independent experiments for each cell line. Statistical significance was determined by Student's t test: *, P < 0.05.
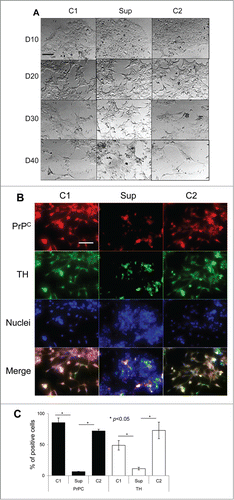
Figure 2. Suppression of PrPC delays differentiation into oligodendrocytes. (A) Phase-contrast images of hESCs with suppressed PrPC-expression (Sup) and in two control lines (C1 and C2) taken at 10th, 20th, 30th, and 40th day of differentiation. (B) Immunostaining for PrPC (red) and Olig1 (green) on 40th day of differentiation. Hoechst 33342 was used for staining of nuclei (blue). Scale bar = 50 μm. (C) Quantification and statistical analyses of PrPC- and Olig 1-positive cells on 40th day of differentiation. The data represent a mean ± SD from three independent experiments for each cell line. Statistical significance was determined by Student's t test: *, P < 0.05.
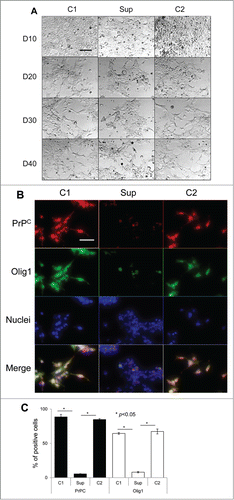
Figure 3. Suppression of PrPC delays differentiation into astrocytes. (A) Phase-contrast images of hESCs with suppressed PrPC-expression (Sup) and in two control lines (C1 and C2) taken at 10th, 20th, 30th, and 40th day of differentiation. (B) Immunostaining for PrPC (red) and GFAP (green) on 40th day of differentiation. Hoechst 33342 was used for staining of nuclei (blue). Scale bar = 50 μm. (C) Quantification and statistical analyses of PrPC- and GFAP-positive cells on 40th day of differentiation. The data represent a mean ± SD from three independent experiments for each cell line. Statistical significance was determined by Student's t test: *, P < 0.05.
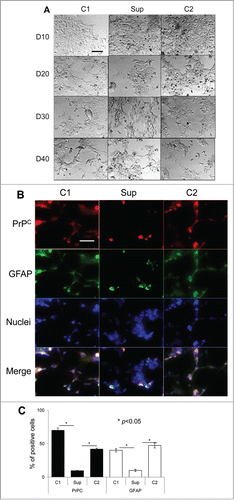
Figure 4. western blotting analysis of expression of PrPC and three marker proteins. Western blotting (A) and quantification with statistical analyses (B) of the expression level of PrPC, Syn, Olig 1, and GFAP in hESCs with suppressed PrPC expression (Sup) and in two control lines (C1 and C2) cultured for 40 d under lineage-preferred differentiation conditions in the presence of tetracycline. β-actin was used as a loading control in (A). In (B) the expression level of each protein was normalized relative to that of β-actin in each differentiation protocol. The data represent a mean ± SD from three independent experiments. Statistical significance was determined by Student's t test: *, P < 0.05.
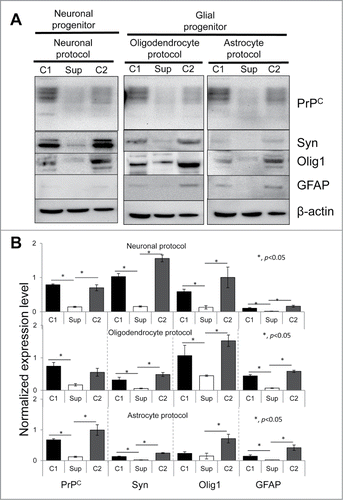
Figure 5. The effect of PrPC silencing at different time points on differentiation of hESCs into neuron-, oligodendrocyte-, and astrocyte-specific lineages. (A) Schematic representation of eight experimental formats, where PrPC was temporarily silenced for 5, 10, or 15 d. hES+TetR+ShPrPC (Sup) cells were cultured under spontaneous differentiating conditions either in the absence (gray arrows, PrPC expression is on) or presence of 1 μg/ml tetracycline (white arrows, PrPC expression is silenced). Western blotting (B) and normalized expression level (C) of PrPC, Syn (synapsin I), Olig 1 and GFAP in hESCs after 15 d of culturing according to eight experimental formats described in panel A. β-actin was used as a loading control. In (C), the expression level of each protein was normalized relative to that of β-actin in each differentiation format. The data represent a mean ± SD from three independent experiments.
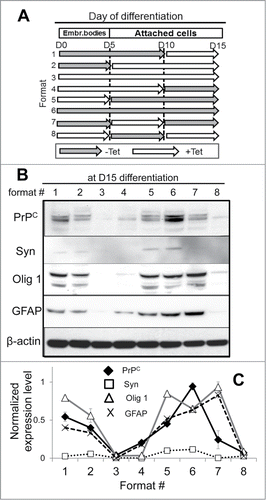
Figure 6. A schematic model illustrating PrPC effects on self-renewal and differentiation of hESCs into neural lineages. Expression of PrPC in pluripotent hESCs triggers their differentiation into neuron-, oligodendrocyte- and astrocyte-committed lineages. PrPC guides differentiation at the very early stage, presumably the stage of uncommitted progenitor, via a yet unknown signaling pathway or through inhibiting cell-renewal.