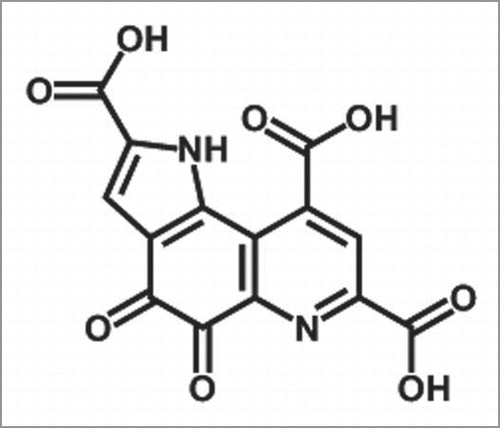
Figures & data
Figure 2 Inhibitory effect of PQQ on the fibril formation of amyloid β (1–42). (A) The time courses of amyloid fibril formation of Aβ1–42 as determined by ThT fluorescence assay analysis. No additive (50 µM Aβ1–42) (white circles), +100 µM PQQ (gray triangles), +300 µM PQQ (black triangles). The sigmoidal curve analysis was performed by PRISM (GraphPad Software). (B) AFM revealed the formation of amyloid fibrils, with short fibrillar morphology, having a diameter of 6∼8 nm and a length of 0.5∼1 µm (Left; 50 µM Aβ1–42). In the presence of PQQ, there are amorphous aggregates having a diameter of 4∼6 nm and length of 10∼500 nm were detected (Right; 50 µM Aβ1–42 + 300 µM PQQ). Scale bars: 1 µm. All images are a height mode. (C) The effect of PQQ on the aggregation of Aβ1–42 was monitored by light scattering at 500 nm after 20 h incubation, n = 2. 50 µM Aβ1–42 (white bar), 50 µM Aβ1–42 + 100 µM PQQ (gray bar), 50 µM Aβ1–42 + 300 µM PQQ (black bar).
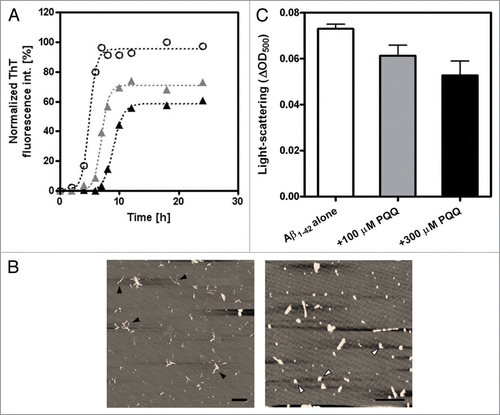
Figure 3 Effect of PQQ on cytotoxicity induced by amyloid β (1–42). Percentage of cell viability of PC12 cells added Aβ1–42 incubated with or without PQQ from n = three independent experiments. The results are expressed as a percentage of the value of “control” (PBS) (gray bar), which is set as 100%: 10 µM PQQ only, 5 µM Aβ1–42, 5 µM Aβ1–42 + 10 µM PQQ. Values are expressed as mean ± SD. (t-test, *p < 0.005).
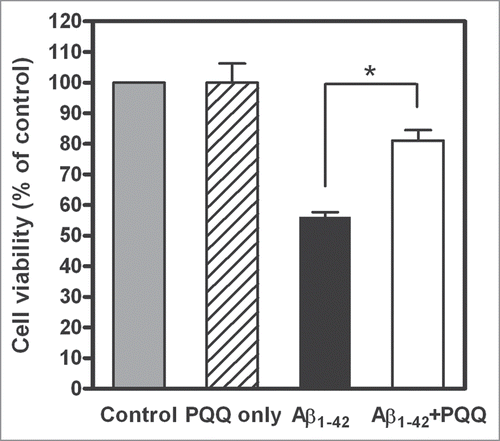
Figure 4 Inhibitory effect of PQQ on the fibril formation of mouse prion. (A) The time courses of amyloid fibril formation of mouse PrP as determined by ThT fluorescence assay analysis. No additive (4.1 µM rPrP) (white circles), +10 µM PQQ (gray triangles), +50 µM (gray diamonds), +100 µM (black triangles), +200 µM PQQ (black diamonds). The sigmoidal curve analysis was performed by PRISM (GraphPad Software). (B) In the AFM observation of PrP, it showed the typically fibrils, having a diameter of 10 nm and a length of 2∼3 µm (Left; 5 µM mouse PrP). However, there are small aggregates observed in the presence of PQQ (Right; 5 µM mouse PrP + 440 µM PQQ). Scale bars: 1 µm. All images are a height mode.
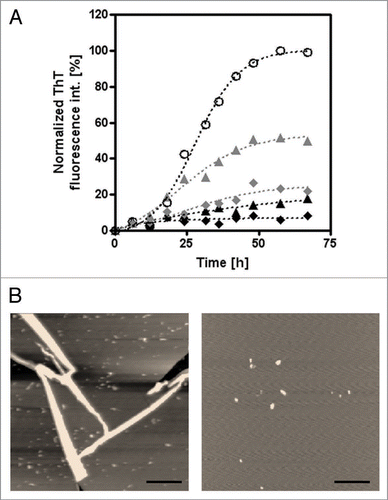
Figure 5 Efficiency of PQQ’s Inhibitory activity depending on amyloid proteins. The final fluorescence intensity of ThT was normalized as a percentage of the value of control which means amyloid protein only as 100%. [PQQ]/[Protein] was the molar ratio of PQQ to each amyloid proteins; α-Syn WT (black circles), Aβ1–42 (black diamonds) and PrP (black triangles). The data of α-Syn used here was from ref. 11.
![Figure 5 Efficiency of PQQ’s Inhibitory activity depending on amyloid proteins. The final fluorescence intensity of ThT was normalized as a percentage of the value of control which means amyloid protein only as 100%. [PQQ]/[Protein] was the molar ratio of PQQ to each amyloid proteins; α-Syn WT (black circles), Aβ1–42 (black diamonds) and PrP (black triangles). The data of α-Syn used here was from ref. 11.](/cms/asset/fa9f7bd4-c3ca-4554-b8ef-c64905b65b04/kprn_a_10910889_f0005.gif)