Figures & data
Figure 1 The binding site of the copper ion at high Cu concentration. Copper is shown in gold, oxygens are red, nitrogens blue, hydrogen white and carbons are cyan.
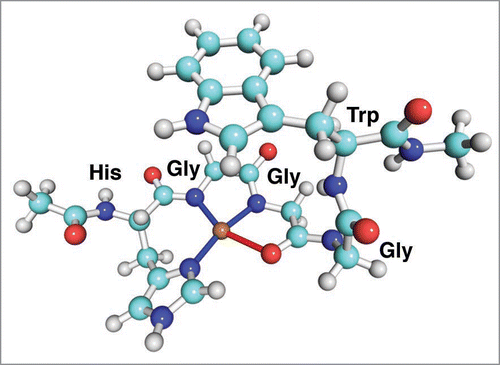
Figure 2 The structure of the low concentration copper binding site in the octarepeat domain of PrP.
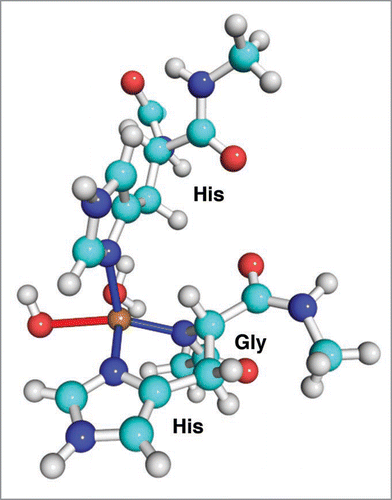
Figure 3 The structure of the medium concentration copper binding mode in the octarepeat domain of PrP.
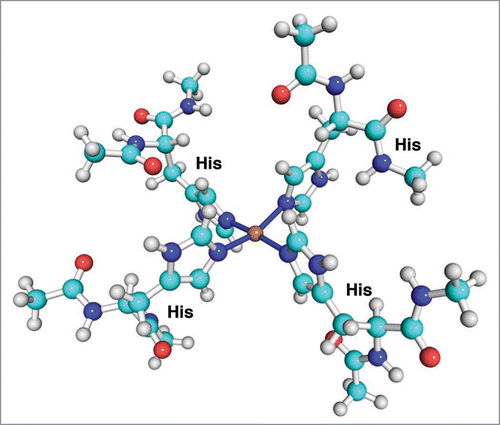
Figure 4 Structures of full-length prion protein in various copper binding modes. (A) Free PrP, (B) Low concentration mode, one copper ion coordinated by His61, His69, His77 and His85, (C) medium concentration mode: two copper ions are attached to His61, His69 and His77, His85, respectively, (D) High concentration mode: four copper ions are attached, each ion is anchored by one of the following: His61, His69, His77 and His85. The copper atoms are shown as gold spheres. The octarepeat domain is colored blue, the non-octarepeat region of the N-terminal is colored grey. The α-helices and β-sheet in C-terminal are displayed in red and mangenta, respectively.
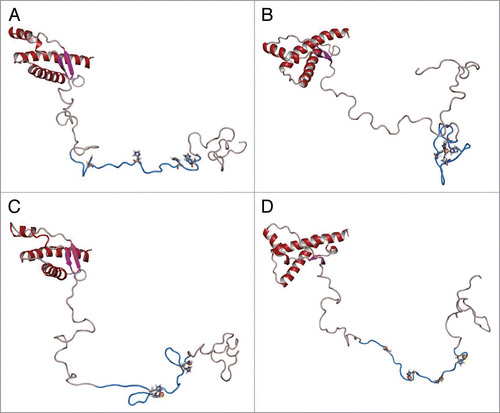
Table 1 Energies of copper binding modes for octarepeat domain of PrP