Figures & data
Figure 1 The effect of fibril fragmentation on length, seeding capability, capacity to cause membrane disruption and cytotoxic potential of fibril samples. (A) Change in weight average fibril length as measured using TM-AFM single particle image analysisCitation18 (upper), ability to disrupt a model lipid membrane formed from 80% (w/w) phosphatidyl choline and 20% (w/w) phosphatidyl glycerol (middle), and initial fibril extension rate when added to fresh monomer solutions (lower) as function of the length of time a preformed β2m fibril sample was fragmented by stirring at 1,000 r.p.m. For the initial fibril extension rate (lower) and dye release efficiency (middle), calculated curves based on a model where the observed changes are directly proportional to the relative increase in the number of fibril particles as fibrils fragment (dashed lines), or best fit non-linear power-law relationship (solid lines) are also shown in each case. The difference in the two curves in the case of dye release efficiency illustrates that membrane disruption follows a more complex relationship with fibril length than fibril extension rate, in a manner that cannot be described solely by the increase in the number of fibrils as fragmentation proceeds. (B) Ability to disrupt liposome membranes (dye release assay), and to decrease cell viability (MTT assay) by samples containing short (white bars) or long (grey bars) fibrils of lysozyme (upper plot), α-synuclein (center plot) or β2m (lower plot). Relative dye release efficiency and relative cell viability were normalised against the effect of long fibrils for comparison. The error bars represent one standard error. Negative stain transmission electron micrographs of the fibril samples (top: lysozyme, centre: α-synuclein, bottom: β2m) are shown to the right, with scale bars representing 200 nm. In each case, the fragmented sample is shown below its unagitated counterpart. (Data were originally published in The Journal of Biological Chemistry, Xue et al. Fibril fragmentation enhances amyloid cytotoxicity. J Biol Chem 2009; 284:34272–82.Citation17 Copyright, the American Society for Biochemistry and Molecular Biology).
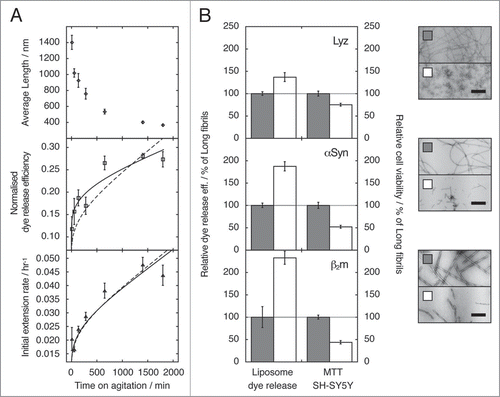
Figure 2 Schematic illustration of the landscape of fibril assembly and fragmentation in relation to the mechanism of fibril associated cytotoxicity. An assembly landscape is illustrated by fibril load plotted against fibril length (upper). The intensity of the red background colour represents the cytotoxic potential. The thick red arrow (I) in the landscape illustrates a representative fibril assembly pathway that would occur in the presence of fibril fragmentation or where nucleation is rapid relative to elongation, resulting in a rapid formation of fibrils with short length distributions. The presence of these short fibrils could lead to enhanced cytotoxicity through decreased fibril-fibril interactions (1) and/or increased fibril-membrane interaction (2). The increased interaction between short fibrils and membrane surfaces could result in membrane damage and a cytotoxic response by fibrils penetrating the membrane (3), growing on the membrane surface (4) or releasing cytotoxic species (5). The thin blue arrow in the assembly landscape (II) illustrates a representative fibril assembly pathway in which little fibril fragmentation occurs or where nucleation is slow relative to elongation, which results in a slow increase of fibril load and the formation of long fibrils. These long fibrils are likely to be less biologically available through increased fibril-fibril interactions (6), decreased interaction with membranes (7) and/or decreased ability of fibrils to pass through the membrane or be taken up by a cell (8) compared with their short counterparts. Fragmentation post-assembly (either mechanical or via chaperones) shortens the average fibril length and thereby enhances the cytotoxic potential without changes to fibril structure (blue to red horizontal arrows III). (Figure was originally published in The Journal of Biological Chemistry, Xue et al. Fibril fragmentation enhances amyloid cytotoxicity. J Biol Chem 2009; 284:34272–82.Citation17 Copyright, the American Society for Biochemistry and Molecular Biology).
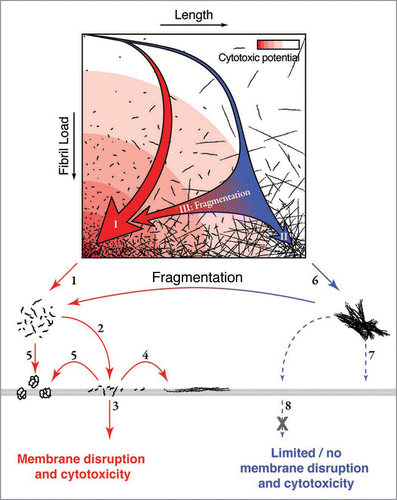
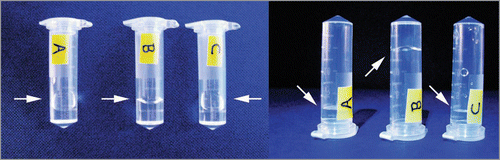
Figure 3 Photographs of a tube with sample solution containing (A) water, (B) unagitated long-straight β2m fibrils displaying gel-like properties and (C) long-straight β2m fibrils that have been fragmented to an average length of ∼400 nm through mechanical stirring. Sample tubes are shown lying down (left photo) or standing in an up side down position (right photo). An arrow indicates the position of the meniscus in each case. The unagitated fibril sample (B) is demonstrating gel-like properties as the meniscus does not follow the tilt of the sample tube (left), nor does the solution readily flow compared with water or fragmented fibril sample (right) likely due to increased viscosity.