Figures & data
Figure 1 Fibrilization of ovine PrP, monitored by thioflavin T fluorescence, in the presence of subcellular fractions of murine LD9 cells. (A) Fraction 1 (B) Fraction 2 (C) Fraction 10 (D) Bar chart showing lag times to fibrilization of ovine PrP in the presence of all 22 fractions produced by fitting sigmoidal curves to raw ThT fluorescence data. Reactions supplemented with fractions 2–5 did not fibrilize completely in the time frame of the assay (24 h) hence sigmoidal cures could not accurately be fitted to allow lag times to be calculated. The same fractions (2–5) were previously shown to enhance conversion of PrP to a protease-resistant isoform in the cell-free conversion assay. The +ve control relates to fibrilization reactions to which no subcellular fractions were added.
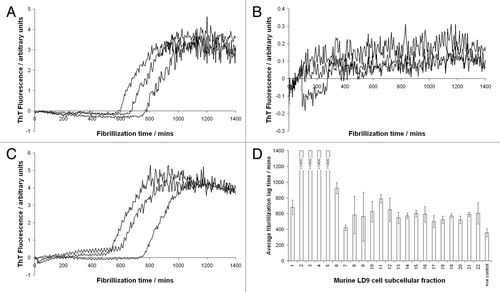
Figure 2 The effect of guanidinium hydrochloride concentration on fibrillization of murine PrP. Guanidine in the final reaction was titrated and lag times to fibrillization of either unseeded (white bars) or seeded (filled bars) reactions were calculated by fitting sigmoidal curves to raw ThT fluorescence data. For unseeded reactions at low guanidine concentrations the protein did not fibrillize to completion in the timeframe of the assay (24 h) hence lag times could not be calculated.
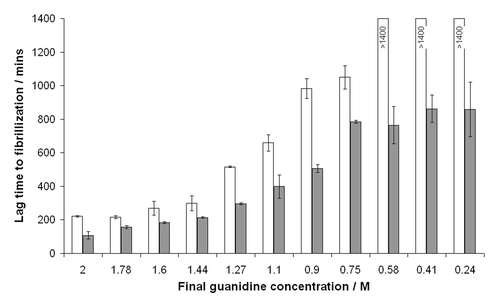
Figure 3 Schematic of a proposed, simplified pathway of PrP folding. Partial denaturation to an intermediate conformation promotes generic misfolding to oligomers and fibrils. Disease-specific misfolding proceeds from a more fully folded form, possibly by route of a different, PrPSc-induced, partially folded intermediate. General conditions that promote unfolding will lead more rapidly to generic misfolded isoforms, while those that promote stability and a more structured PrPC molecule will favor disease-specific misfolding.
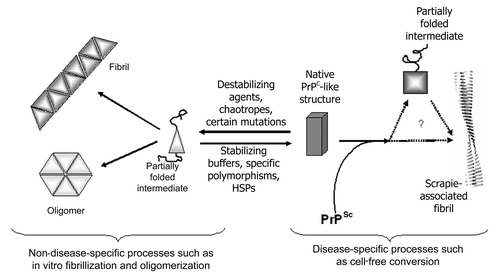
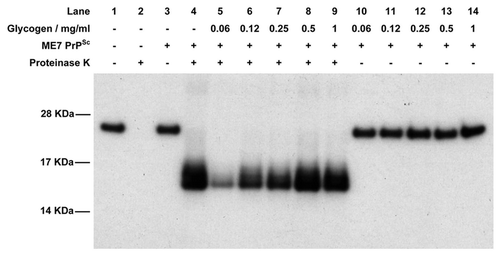
Figure 4 Western blot of the cell-free conversion assay of 3F4 antibody-tagged murine recombinant PrP in the presence of glycogen. Assays were seeded with PrPSc purified from ME7-infected mice and, in each case, 9/10th of the reaction was treated with PK while the remaining 1/10th was not. In the absence of PrPSc, recombinant PrP is digested away by PK (lanes 1 and 2) while in the presence of PrPSc a protease-resistant isoform is produced (lanes 3 and 4). In the presence of low concentrations of glycogen, cell-free conversion is inhibited (lanes 5–7) while at higher concentrations of glycogen conversion is similar to control levels (lanes 9 and 10). Lanes 11–14 contain the non-PK treated samples in the presence of glycogen to demonstrate that similar levels of recPrP substrate were present in each assay.