Figures & data
Figure 1 The autophagy pathway may converge with the proteasome to produce a powerful protein clearing system named autophagoproteasome. Most of the protein aggregates which occur in the gut mirror analogous structures within CNS. These proteins all share the property to be metabolized preferentially by the autophagy pathway. This powerful protein and organelles clearing system starts mainly in the membrane of the Golgi apparatus and endoplasmic reticulum where it blossoms as a primary structure named the phagophore which becomes committed to the autophagy pathway following the association with the protein LC3-II. This multi-membrane vesicle develops as an autophagosome which is characterized by a double membrane vesicle in which mono or poly ubiquitinated misfolded proteins and/or mitochondria are sequestered. The autophagosome may directly fuse with the lysosome or merge with the late endosome (known also as multi vesicular body) to build up an amphisome which eventually merges with the lysosome. Another protein clearing pathway is the ubiquitin proteasome system which clears only protein substrate upon their polyubiquitination. Despite being described as distinct organelles in the cell, recent experimental evidence indicates that proteasome components and autophagosome converge into a complex clearing organelle in which the proteasome enzymes add on classic autophagy proteins,Citation48,Citation49 which was named the autophagoproteasome.Citation50–Citation52
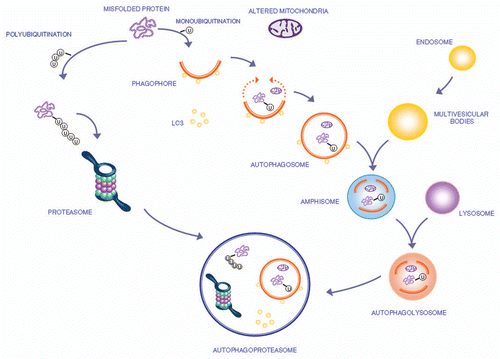
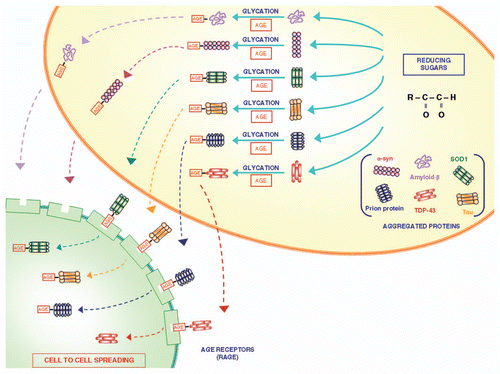
Figure 2 The role of glycation in modifying proteins structure and function. Glycation is a post-translational, non-enzymatic reaction between reducing sugars and amino groups, resulting in the formation of AGE. The major glycation agents include methylglyoxal, glyoxal and 3-deoxyglucosone. Misfolded proteins represent an important target for glycation. A variety of proteins involved in neurodegenerative disorders (such as Aβ, SOD-1, α-synuclein, TDP-43, tau protein and prion protein) undergo glycation, responsible for an increased stability by the formation of crosslinks that stabilize protein aggregates. These AGE are expressed on the plasma membrane and may diffuse extracellularly to interact with neighbouring cells to spread according to a prion-like mechanism.Citation34 In fact, glycated proteins would interact with RAGE, thus allowing a “cell to cell spreading” by their internalization. This process, which is well demonstrated within CNS, remains at hypothetical level within the gut, where in any case the prion protein is shown to spread from cell to cell.