Figures & data
Figure 1 Diagram of the pathway involved in prion- or Aβ-induced dysfunction of memory and cognition as well as cell death. PrPSc or Aβ oligomers impair memory and cognition, and induce cell death by binding to PrPC at the synapse but the downstream molecular events (the black box with a question mark) of the PrPSc-PrPC or PrPC-Aβ complex are unknown. The iPrPC species derived from the soluble PrPC might play a role in one or more of the following events: (1) long-term memory storage; (2) PrPSc formation in classic CJD; (3) initiating VPSPr; and (4) facilitating formation of Aβ42 fibrils in AD.
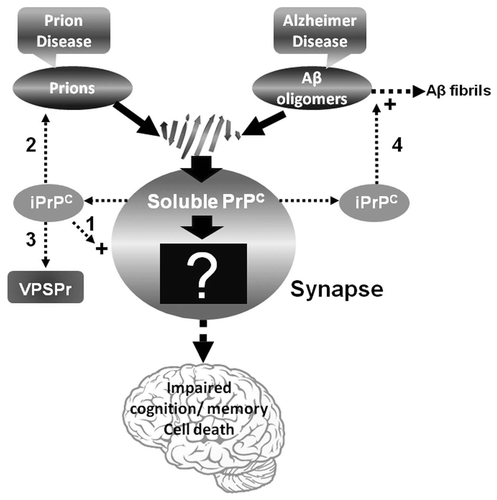
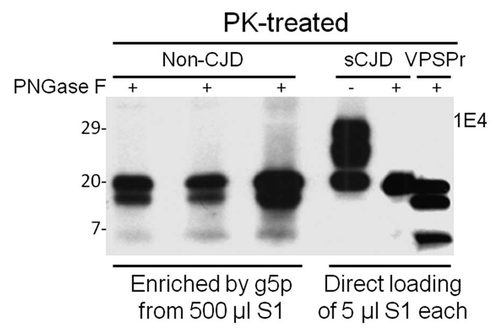
Figure 2 Comparison of PK-resistant PrP core fragments from non-CJD, VPSPr and sCJD. PrP captured with g5p from brain homogenates of three non-CJD subjects was treated with PK and PNGase F prior to SDS-PAGE and immunoblotting with anti-PrP antibody 1E4. Fifty µl of insoluble fraction (P2) equivalent to 500 µl of supernatant (S1) from a low-speed centrifuge was used for each non-CJD subject. In these highly concentrated samples from non-CJD subjects, three PK-resistant PrP (PrPres) fragments migrating at ∼20 kDa, ∼17–18 kDa and ∼6–7 kDa were detected with 1E4. But these PrPres fragments were not detectable by 3F4 (data not shown). In contrast, three PrP fragments with similar gel mobility were also detected in only 5 µl of S1 preparation from a VPSPr case (129MV) by 1E4 after treatment with PK and PNGase F. However, only one PrP band was detected in sCJD samples after PK and PNGase F treatment.