Figures & data
Figure 1 Amino acid sequence alignment of Y145Stop from different species is shown. The alignment was obtained using ClustalW of huY145Stop with the corresponding sequences in cattle, deer and sheep genome.

Figure 2 (A) Expression of full-length prion protein and Y145Stop of sheep and deer. Immunoblot of the purified proteins transfected with the expression vector alone or vectors containing constructs of the designated species (defined in the legend A) shows full degradation when incubated for 1 h in the presence of proteinase K. Positions of the molecular mass markers are designated in kDa. (B) Proteinase K digestion of sheep and deer Y145Stop molecules after PMCA at different time points. Human recombinant PrP 23–231 was used as a positive control. Spontaneously generated fibrillar proteins persisted after proteinase K digestion and were detected at 16 h with more conversion at 24 and 36 h after PMCA incubation. (C) Immunoblotting of PrP-sen of the purified recombinant full-length prion protein of sheep and deer strains; white-tailed deer (G96 and S96 variants) and sheep (171R) after PMCA and PK digestion at different time points. Proteins were fully degraded in the presence of proteinase K with no detectable conversion after 72 h of PMCA.
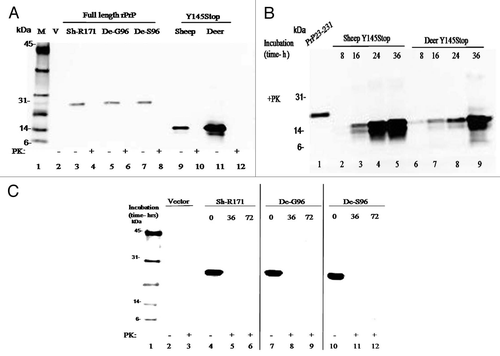
Figure 3 Time-course analysis to document increase in Thioflavine T uptake and fluorescence by sheep and deer Y145Stop in the absence of seeds. (A) ShY145Stop (squares) and full-length Sheep PrP with R at codon 171 (triangles) incubated in the absence of seeds. (B) DeY145Stop (circles) and full length Deer PrP with G (squares) or S (triangles) at codon 96 incubated with no seeds. All samples were subjected to PMCA under identical conditions. ShY145Stop and DeY145Stop acquired the ability to bind ThT without addition of external seeds. In contrast, the full-length prion protein did not show detectable ThT binding. The kinetic curves were characterized by a lag phase (8–12 h).
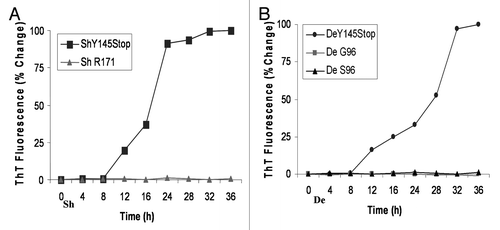
Figure 4 The progress of amyloid fibril formation measured by fluorimetric Thioflavin T uptake assay in presence of seeds. One percent (w/w) performed fibrillar seeds (denoted in square brackets) were added to solutions containing monomers of sheep (A) or deer (B) Y145Stop. No seed control showed the characteristic lag phase, while seeded reactions with partially purified PrPSc from deer [CWD] or sheep [Sc] eliminated the lag phase. Similar conversion rates were observed with cross seeding by in-vitro converted Y145Stop of sheep [ShY145Stop] or deer [DeY145Stop].
![Figure 4 The progress of amyloid fibril formation measured by fluorimetric Thioflavin T uptake assay in presence of seeds. One percent (w/w) performed fibrillar seeds (denoted in square brackets) were added to solutions containing monomers of sheep (A) or deer (B) Y145Stop. No seed control showed the characteristic lag phase, while seeded reactions with partially purified PrPSc from deer [CWD] or sheep [Sc] eliminated the lag phase. Similar conversion rates were observed with cross seeding by in-vitro converted Y145Stop of sheep [ShY145Stop] or deer [DeY145Stop].](/cms/asset/55412f3f-04b5-470b-89ef-bf1a237c7ad2/kprn_a_10918493_f0004.gif)
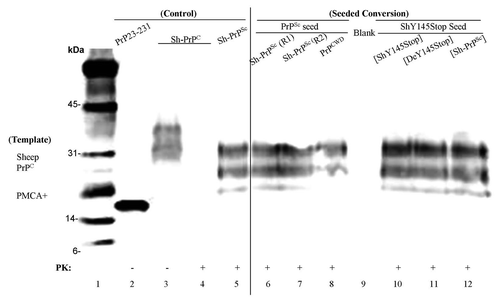
Figure 5 PMCA on purified PrPC from brain tissue of sheep and deer. Lane 1, molecular weight marker (biotinylated). Lane 2, human recombinant PrP 23–231 was used as a positive control. Lanes 3 and 4, samples containing purified PrPC from brain tissue of sheep before and after PK digestion. Lane 5, PrPSc extracted from sheep brain as a reference after PK digestion with 10 times the reaction equivalents of the seed protein concntration. Samples of purified PrPC from brain tissue of sheep were incubated with preformed fibrillar seed of PrPSc round one (lane 6), round two (lane 7), PrPCWD (lane 8), ShY145Stop seeded with performed fibrils of ShY145Stop (lane 10), DeY145Stop (lane 11), or PrPSc (lane 12) after PK digestion.