Figures & data
Figure 1. A schematic model of ethylene biosynthesis showing a few of its components as affected by NO during fruit ripening. (Straight arrows indicate established phenomena; dotted arrows indicate phenomena of unidentified mechanisms). Ethylene is perceived by a family of five membrane bound receptors (ETR1, ETR2, ERS1, ERS2 and EIN4) which are characterized having a sensor and a response regulator domain. In the absence of ethylene, the receptors activate the kinase activity of CTR1 (constitutive triple response 1) a negative regulator that suppresses downstream progression of signaling. CTR1 then actively suppresses the downstream responses, such that EIN2 and the EIN3/EIL family of transcription factors remain inactive. Upon perception of ethylene, the receptors no longer activate CTR1, thus activating the EIN3/EIL family of transcription factors. Ethylene insensitive 3 (EIN3) target is thought to be the ethylene response factor 1 (ERF1) gene. ERF1 encodes an ethylene response element binding protein (EREBP) that binds the GCC-box, a cis-element of many ethylene response genes, thus activating the ethylene-signaling pathway. In the ethylene cascade, autocatalytic activity of ethylene is reported to form SAM (S-adenosyl methionine) from methionine (MT) which is catalyzed by SAM synthetase, whereas SAM is catalyzed into Aminocyclopropane carboxylic acid (ACC) by ACC synthase and further oxidized into ethylene by ACC oxidase. Ethylene biosynthesis in this route was found affected by NO mainly through inhibition of SAM turnover via S-nitrosylation of transcriptionally produced methionine adenosyl transferase (MAT). Further, genes coding for ACCS and ACCO were downregulated (┴) by NO accounting for the reduction of ethylene. Stoichiometric reduction of ACC to 1-melonyl aminocyclopropane 1-carboxylic acid (MACC) and formation of a stable ternary ACC-ACCO-NO complex can also antagonize ethylene formation. Alternatively, reciprocal interaction of NO and hydrogen peroxide (H2O2) were also presumed affecting MAP kinase-mediated downstream components of ethylene biosynthesis. Growth regulators (GRs) and NO may also influence redox status and other signal generation. As a consequence, NO affects the yield of ethylene, thus delaying expressions of enzymes responsible for cell wall degradation, lignification and pigmentation of fruits conferring shelf life extension.
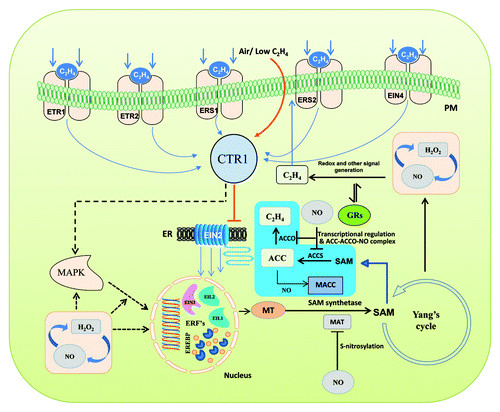
Table 1. Effect of nitric oxide on quality parameters of fruits
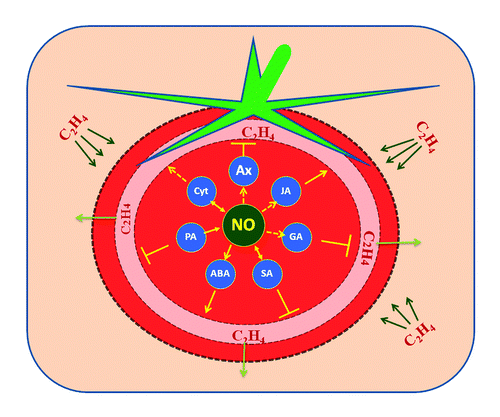
Figure 2. The overlapping interactions of polyamines (PA), jasmonic acid (JA), salicylic acid (SA), auxin (Aux), cytokinins (Cyt), abscisic acid (ABA) and gibberellic acid (GA) that are responsive to ethylene stimuli and their plausible relations with NO during ripening. Although NO's relation with phytohormones (Aux and GA) and signal molecule (JA) are yet to be established, the possible link between the ethylene biosynthesis inhibition (┴) by former and enhancement (→) by the latter can be hypothesized. The reciprocal interactions (↔) of NO with Cyt and SA affect the ethylene turnover during fruit ripening. NO's direct interaction with PA and ABA is known to negatively modulate ethylene biosynthesis. (Straight arrows indicate established phenomena; dotted arrows indicate phenomena of unidentified mechanisms).