Figures & data
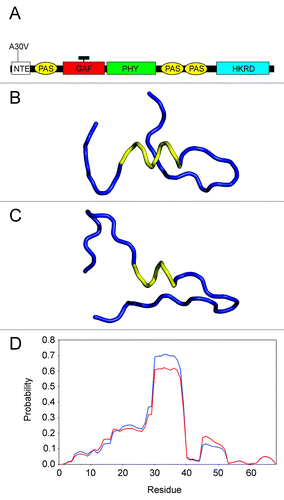
Figure 1. Location and nature of the phyA-5 mutation. (A) Schematic arrangements of the phytochrome domains (not to scale). The position of phyA-5 mutation is indicated. NTE, N-terminal extension; PAS, PER/ARNT/SIM domain; GAF, cGMP phosphodiesterase, adenylate cyclase, FhlA domain; PHY, phytochrome domain; HKRD, histidine kinase-related domain; black rectangle represents the chromophore. (B) Backbone conformation of the predicted structure, based on the sequence including the residues 2050 for the PHYA protein. The helical segment is represented by yellow ribbon, while the other parts of molecule by blue ribbon. (C) Backbone conformation of the predicted structure, based on the sequence covering the residues 2050 for the PHYA-5 protein. The helical segment is represented by yellow ribbon, while the other parts of molecule by blue ribbon. (D) Predicted probabilities regarding the α-helical structure for the amino acids, on the basis of sequence including the residues 168 for the PHYA (blue line) and PHYA-5 (red line) proteins.