Figures & data
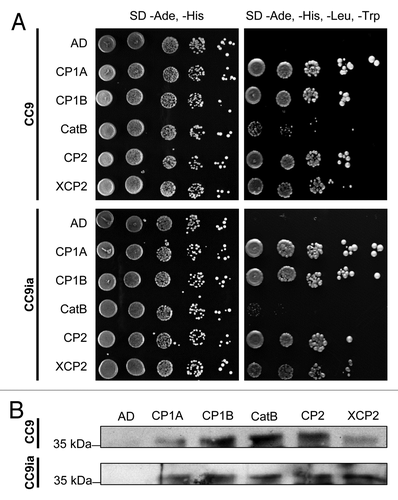
Figure 1.Phylogenetic analysis of cystatins from Zea mays, Oryza sativa and Arabidopsis thaliana. Alignment data of full length proteins from Arabidopsis (AtCys), rice (OC) and maize (CC) cystatins were obtained using clustalw2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/). The unrooted tree was calculated using RAxML BlackBox (http://phylobench.vital-it.ch/raxml-bb/) and was visualized by iTOL (http://itol.embl.de/). 100 bootstraps were performed. Bootstrap values are given in the Figure. Bootstrap-supported clusters of related cystatins (cluster I-III) are marked in shades of gray. CC9 is highlighted in bolt letters.
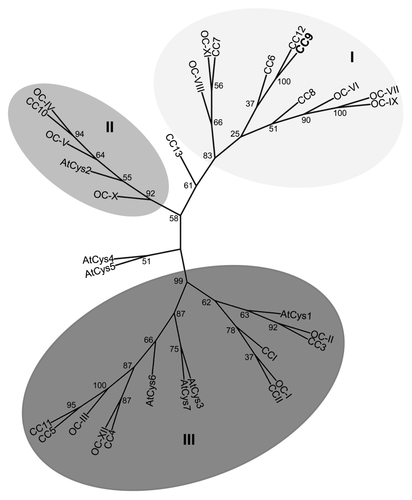
Figure 2. Structural features of the apoplastic maize cysteine proteases. All five apoplastic maize PLCPsCitation10 carry a signal peptide (SP) for endomembrane targeting (gray), an autoinhibitory prodomain (Pro-, dark gray), and a protease domain (bright red), which harbours the catalytic triad (C-H-N, red). Two of the proteases (CP1A, CP1B) also carry a C-terminal proline-rich domain (P, gray) and a granulin domain (purple). Putative N-glycosylation sites (green dots) and disulphide bridges (thin bent lines) in the protease domain are indicated. CP2 also carries a vacuolar targeting signal (NPIR, blue) and a minichain (orange) that remains linked to the protease after cleavage of the Pro-domain.
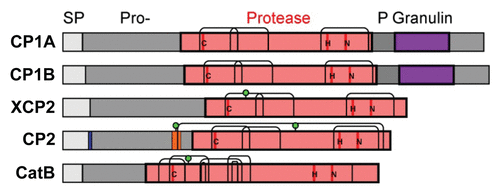
Figure 3. Interaction of CC9 with the apoplastic maize cysteine proteases by Yeast-2-Hybrid and co-immunoprecipitation. A) Yeast-2-Hybrid interaction assay of CC9 and inactive CC9 (CC9ia) with the five proteases. Yeast drop assays after co- transformation of CC9 (CC9ia) fused to binding domain (BD) with CP1A, CP1B, CatB, CP2 or XCP2 fused to activation domain (AD). Growth on low stringency medium (SD -Ade, -His) selects for expression of AD and BD constructs. Growth of all strains on high stringency medium (SD -Ade, -His, -Leu, -Trp) indicates interaction of the co-expressed proteins. B) Yeast strains shown in (A) were used for co-immunoprecipitation experiments. From each yeast strain, 2 mg of total protein were incubated with 50 µl of anti-HA beads (Roche, Germany) to co-immunoprecipitate the HA-tagged proteases with CC9-MYC. The eluted fractions were analyzed by western blotting using an anti-MYC antibody. CC9-MYC (or CC9ia-MYC) was detected at the expected size (37 kDa), demonstrating protein-protein interaction.