Figures & data
Figure 1. NAD-GDH isoenzyme patterns of roots of Arabidopsis wild type and mutants deficient in the three genes encoding the enzyme. (A) Protein extracts of the roots of the wild type (WT), gdh1, gdh2, gdh3, gdh1-2 and gdh1-2-3 mutants were subjected to native PAGE followed by NAD-GDH in-gel activity staining. The different subunit combinations of the eight isoenzymes detected in the WT are indicated on the left side of the panel. Double arrows indicate the shift observed between the gdh2 and the gdh3 mutants for the positions of the different isoenzymes. (B) Protein extracts of the roots of the WT, gdh1 and gdh2 mutants were subjected to native PAGE followed by NAD-GDH in-gel activity staining. The possible combinations of the three GDH subunits α, β and γ in the different isoenzymes, when they are all present in the WT are shown in the table at the right side of the panel. The subunit composition that predominates in the WT is indicated in red (black closed arrows). The red open arrows in the WT indicate the position of the other subunit composition also listed in the table on the right. The possible different combinations of the subunits in the GDH isoenzymes, when the subunit α or the subunit β is lacking in the gdh2 and gdh1 mutants respectively are also shown in blue (black closed arrows) and in the table on the right. The red open arrows in the gdh1 mutant indicate the position of the five heterohexamer isoenzymes and their composition is presented in the table on the right.
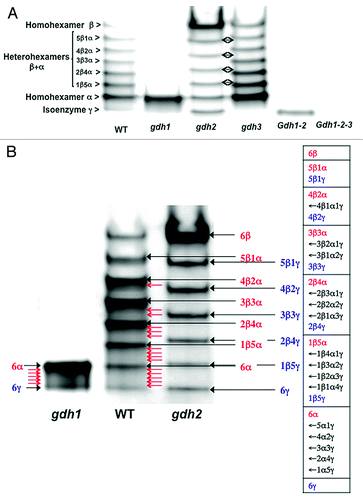
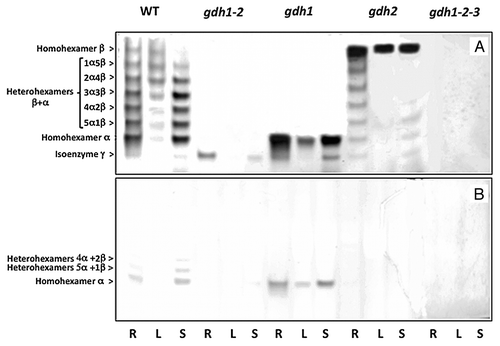
Figure 2. NADH- and NADPH-dependent GDH isoenzyme patterns of roots, leaves and floral stems of Arabidopsis WT and mutants deficient in the three genes encoding the enzyme. Protein extracts of the roots (R), leaves (L) and floral stems (S) of the wild type (WT), gdh1, gdh2, gdh1–2 and gdh1–2-3 mutants were subjected to native PAGE followed by NAD-GDH (A) and NADP-GDH (B) in-gel activity staining. The composition of the subunits of the eight isoenzymes in the WT are indicated on the left side of the panel.