Figures & data
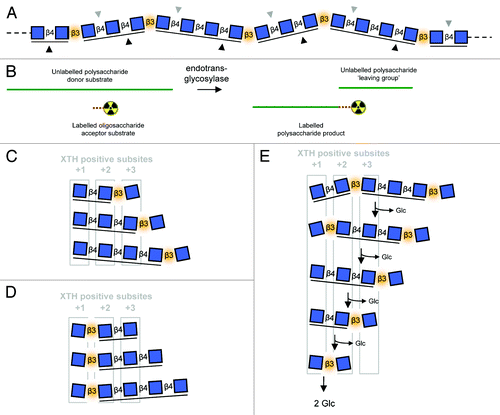
Figure 1. Endotransglycosylase reactions and the use of MLGOs as acceptor substrates. (A) MLG polymer structure showing kinks caused by β-(1→3) bonds as well as distinct sites of enzymic attack: lichenase cleaves β-(1→4) bonds after β-(1→3) bondsCitation29 (gray downward triangles), cellulase cleaves β-(1→4) bonds before β-(1→3) bondsCitation30 (black upward triangles); (B) General endotransglycosylase reaction. During assays the acceptor substrate is typically a labeled (e.g., radioactive) oligosaccharide. Labeled polymeric material is detected after the reaction; Major products of lichenase (C) and cellulase (D) digestion of MLG as each might fit into XTH positive (acceptor substrate-binding) subsites; (E) 5 successive β-D-glucosidase digestion reactions (each releasing Glc from the non-reducing terminus) on a MLG-hexasaccharide (produce by incomplete lichenase digestion) demonstrating the alternation of position of the β-(1→3) kink relative to XTH positive subsites. Reducing termini on right; blue squares, D-Glc residues; β3, β-(1→3)-linkage (highlighted in orange); β4, β-(1→4)-linkage; cello-like β-(1→4)-linked regions underlined.