Figures & data
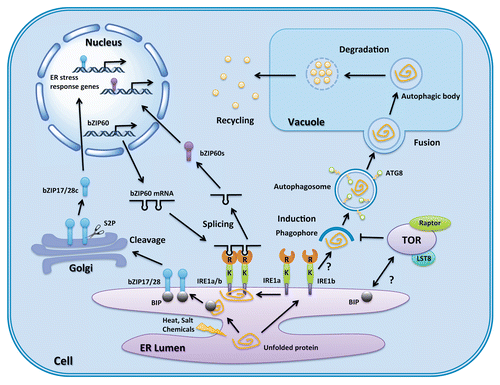
Figure 1. Autophagy and ER stress regulation pathways in plants. A general schematic of autophagy and the UPR under ER stress is shown. ER stress is caused by disturbance of protein folding, and two branches of the UPR are induced by unfolded or misfolded protein aggregates in the ER lumen. The first pathway starts when BIP recognizes and binds to unfolded or misfolded proteins, which activates bZIP17 or bZIP28 to translocate to the Golgi and be cleaved by S2P. The cleaved bZIP17 or bZIP28 enters the nucleus and initiates ER stress response gene transcription. The second pathway begins with oligomerization of IRE1 and its binding to unfolded or misfolded protein. The activated IRE1 splices bZIP60 mRNA. Spliced bZIP60 mRNA is translated and enters the nucleus to begin transcription of ER stress response genes. IRE1b is also required for autophagy induction, although how it is involve in the activation process is still unknown. ATG8 is a key protein for autophagosome formation, and is also used as a marker to monitor autophagy. Autophagosomes then carry cargo to the vacuole where the outer membrane fuses with the vacuole membrane, and the inner membrane and cargo are degraded in the vacuole. Breakdown products are exported into the cytosol for reuse. bZIP17/28c, cleaved form of bZIP17 or bZIP28; bZIP60s, translated protein from IRE1-spliced bZIP60; R, ribonuclease domain of IRE1; K, kinase domain of IRE1; S2P, golgi-associated site 2 protease.