Figures & data
Figure 1. Subcellular localization and molecular interactors of Arabidopsis IQD1. (A) Transient expression of CaMV 35SPro:IQD1~GFP in tobacco leaves (N. benthamiana) reveals association of GFP fluorescence with the microtubular network and cell nucleus (epidermal cell in the center). Lower IQD1~GFP expression level (upper cell) or lower photomultiplier gain (inset, lower right corner) indicates targeting of IQD1~GFP to the nucleolus (see ref. Citation26 for original report and controls). (B) In addition to its recruitment to microtubules, IQD1 interacts in planta with Arabidopsis CaM2 and Arabidopsis KLCR1.Citation27 A possible interaction of IQD1 in planta with GSTU26 (reproducibly identified in a yeast two-hybrid screenCitation27) and with single nucleic acid substrates (demonstrated in vitro),Citation27 such as cellular RNAs, remains to be tested. Querying the PhosPhAt4.0 databaseCitation45 retrieved evidence for in vivo phosphorylation (-P) of IQD1on a site near to its N-terminus. To date, there is no evidence for binding of KLCR1 to kinesin motor proteins, which facilitate cellular transport of specific cargo along microtubular tracks.
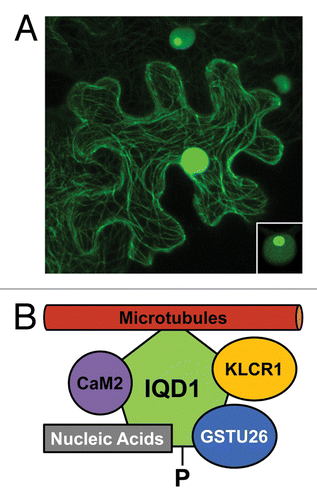
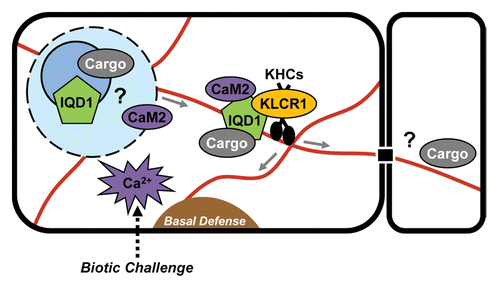
Figure 2. Current model of IQD1-regulated cellular processes. IQD1 is hypothesized to function as a scaffold protein that recruits in a Ca2+-CaM-dependent manner various cargos to kinesin (KHCs) motor complexes via KLCR1. Proteins such as GSTU26 or as yet unidentified RNAs are putative cargo molecules. Ribonucleoprotein complexes containing IQD1 may be assembled in the cell nucleolus/nucleus, exported and directionally transported along microtubule tracks to specific cellular sites related to plant defense response for local mRNA translation, or via plasmodesmata to adjoining cells. A generalized model may apply to other IQD family members.