Figures & data
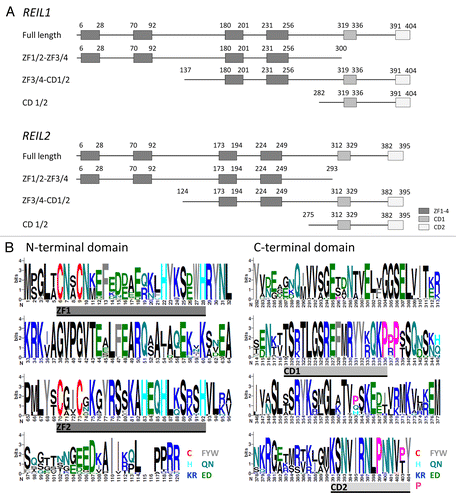
Figure 1. The cytosolic maturation machinery of the 60S large ribosomal subunit from Saccharomyces cerevisiae. The flow scheme represents the current model of the cytosolic maturation machineryCitation5 starting with the pre-60S subunit after export from the nucleus (top) and ending with the translational active 60S ribosomal subunit (bottom). The direct mechanistic interaction partners of the yeast Rei1 protein within the model are indicated by light gray underlay. The cytosolic maturation releases inhibitory factors, such as the Arx1/Alb1 complex, Tif6, and Nmd3, (main path on the left) and enables assembly of the ribosome stalk (side branch on the right).
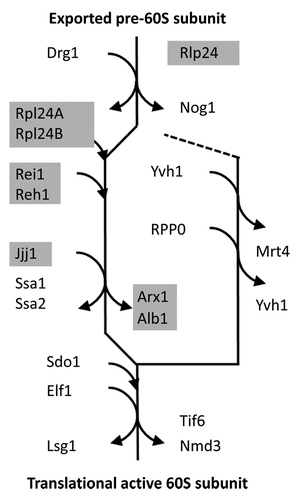
Figure 2. Protein–protein interaction matrix of the cytosolic maturation machinery of the 60S large ribosomal subunit from Saccharomyces cerevisiae. Each dark gray box within the matrix indicates a physical interaction between the cytosolic maturation factors. Interactions were retrieved and consolidated from BioGRID (Version 3.2.106). The protein names, corresponding gene codes, and the total number of physically interacting proteins are listed in the first 3 rows. The rows and columns are symmetrically ordered according to the sequence of the proteins acting within the main path of the model followed by the proteins relevant for stalk assembly, namely RPP0, Mrt4, and Yvh1. The interactions of the Rei1 protein are highlighted by a framed column. The light gray underlay accentuates the direct mechanistic interaction partners of Rei1, also refer to ().
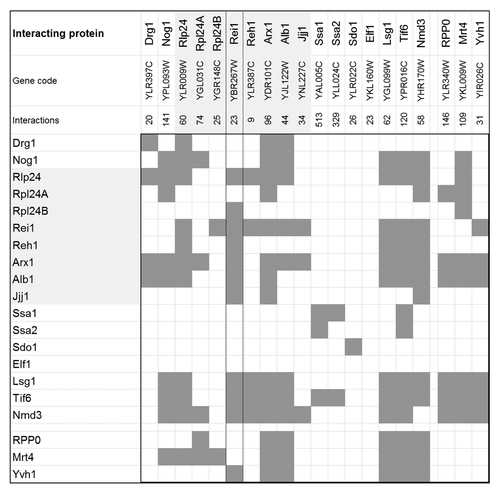
Figure 3. Topology of the REIL (Rei1-like) proteins from A. thaliana and of the partial proteins which were analyzed in this study by yeast-2-hybrid assays ().(A) The 404 amino acid REIL1 and the 395 amino acid REIL2 protein each contain 4 zinc finger domains and 2 additional conserved domains, provisionally named CD1 and CD2. The truncated proteins, ZF1/2-ZF3/4, contain the 4 zinc finger domains. The CD 1/2 truncations contain only the C-terminal parts with the conserved domains CD1 and CD2. The ZF3/4-CD1/2 partial proteins lack the N-terminal pair of zinc finger domains. (B) N-terminal and C-terminal alignment of REIL1 and REIL2 homologs of Brassicales species. Zinc finger domains, ZF1 and ZF2, and conserved domains, CD1 and CD2, are indicated. The color coding of the sequence logos highlights cysteine and histidine residues, aromatic amino acids, amide, acidic, basic residues, and the conserved proline residues of the C-terminus. Color coding is given with the insert. Sequence logos were generated at http://weblogo.berkeley.edu/logo.cgi after alignment of the REIL proteins from Arabidopsis thaliana, Arabidopsis lyrata subsp. lyrata, Eutrema parvulum, Eutrema salsugineum, Brassica rapa subsp. pekinensis, Capsella rubella, Camelina sativa, Leavenworthia alabamica, Sisymbrium irio, and Aethionema arabicum.