Figures & data
Figure 1 Tonoplast H+-ATPase (V-ATPase) activity and plasma membrane H+-ATPase (P-ATPase) activity in wild type Arabidopsis (ecotype Col-0) and Arabidopsis lines with manipulated tonoplast Ca2+/H+ exchange activity. 35S::CAX1 and 35S::CAX2 denote lines that overexpress a constitutively active N-terminally truncated CAX1 or CAX2 construct driven by the CaMV 35S promoter in the cax1-1 or cax2-1 mutant background, respectively. V-ATPase H+-transport activity was measured by the ATP-dependent quenching of quinacrine fluorescence, and rates of bafilomycin-sensitive, vanadate-resistant hydrolytic activity of the V-ATPase were determined in isolated tonoplast membranes, as described in refs. Citation11 and Citation13. Rates of vanadate-sensitive, bafilomycin- and azide-resistant hydrolytic activity of the P-ATPase were determined in isolated plasma membranes, as described in ref. Citation14. Results are shown as % of wild type (Col-0) ATPase activity and are means ± SE of 3–4 independent experiments. Data taken and modified from refs. Citation11–Citation14.
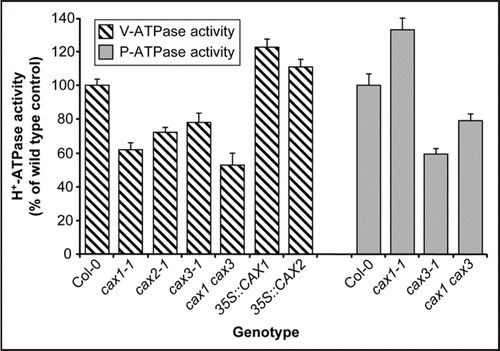
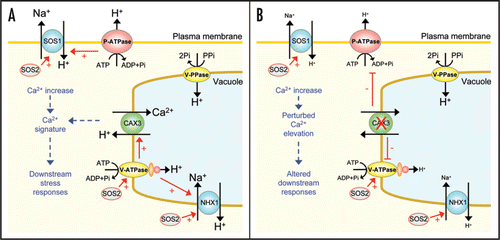
Figure 2 Model of tonoplast Ca2+/H+ exchanger interaction with H+ pumps in response to salt stress. (A) In response to NaCl treatment, an elevation in cytosolic Ca2+ will occur, possibly due to vacuolar Ca2+ release.Citation3 Increased CAX3-mediated Ca2+/H+ exchange activityCitation14 will sequester excess Ca2+ into the vacuole. CAX3 may be involved in the generation of a specific Ca2+ signature that is recognised by the cell to mediate downstream stress responses. In addition, salt stress will lead to upregulation of H+ pumps at both the plasma membrane and the tonoplast (P-ATPase and V-ATPase)Citation25 which will in turn energize Na+/H+ exchange activity encoded by SOS1 and NHX1, promoting Na+ efflux from the cell. Increased V-ATPase activity may also upregulate Ca2+/H+ exchange. Activity of SOS1 requires activation by the kinase SOS2Citation24 which may also regulate tonoplast Na+/H+ exchange and V-ATPase activity.Citation23,Citation24 (B) In a cax3 knockout mutant experiencing salt stress, the cytosolic Ca2+ elevation may be sustained due to reduced vacuolar Ca2+ sequestration and normal salinity-induced Ca2+ signalling pathways may be perturbed. Lack of CAX3 downregulates both P-ATPase and V-ATPase activityCitation14 thereby reducing energization of the plasma membrane and tonoplast Na+/H+ exchangers and reducing Na+ efflux from the cell. Some energization of H+-coupled processes at the vacuole may be maintained by residual H+-pyrophosphatase (V-PPase) activity.