Figures & data
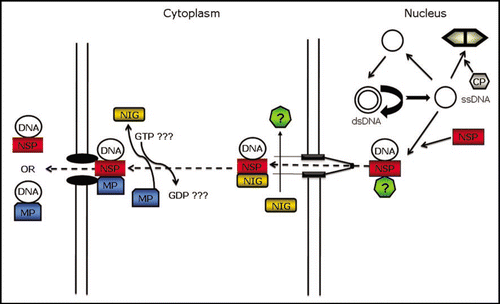
Figure 1 Proposed model for viral DNA intracellular trafficking. Geminiviruses replicate their circular, single-stranded DNA genomes via double-stranded DNA intermediates in nuclei of infected cells. During rolling-circle replication of viral DNA, the newly synthesized single-stranded DNA (ssDNA) may either re-enter the replication cycle, or be sequestered by the coat protein (CP) or NSP in the case of bipartite begomoviruses. Binding of NSP to viral DNA facilitates the intracellular movement of the viral genome from the nucleus to the cytoplasm via an exportin-like receptor (?). At the cytosolic side of the nuclear pore complex, NIG binds to NSP and redirects the viral DNA-NSP complex to the cell periphery where is replaced by MP. Thus, NIG may provide the directionality for the intracellular movement of the newly synthesized viral DNA. The NIG GTPase activity may regulate the assembly and/or disassembly of NIG-based complexes. MP either interacts directly with and transports the viral DNA or mediates the transport of the NSP-DNA complex to adjacent cells via plasmodesmata.