Figures & data
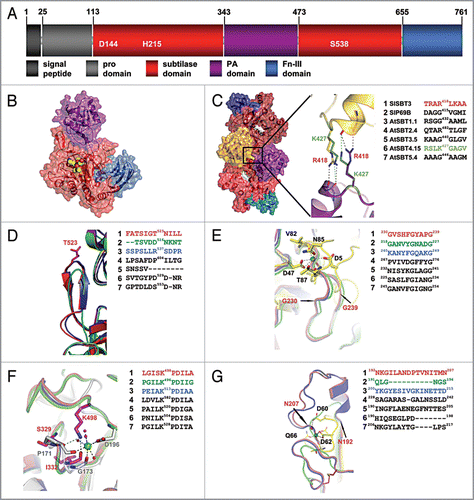
Figure 1 Structural comparison of plant subtilases. (A) Domain architecture of SlSBT3. In addition to the domain borders the three residues constituting the active site are displayed. (B) Structure of the SlSBT3 monomer. Color coding of the domains is like in (A). The bound chloromethylketone (cmk)-inhibitor is shown as ball model in yellow (carbon), red (oxygen) and blue (nitrogen). (C) Functional homodimer of SlSBT3. The region of the direct contact between the two PA domains (gold, purple) is highlighted. In this and all the following panels, a sequence alignment of the relevant regions in SlSBT3 and the modeled subtilases is shown on the right. The sequences highlighted in color were included in the structural alignment on the left. (D) Structure of the partially conserved β-hairpin. (E) Structure of the region corresponding to the conserved calcium-binding site 1 (Ca-1) in thermitase (yellow sticks, PDB code: 1THM). (F) Functional substitution of the conserved Ca-2 (white sticks, PDB code 1S2N) site by a lysine side chain in plant subtilases. (G) Structure of the region corresponding to the less conserved Ca-3 site in thermitase (yellow sticks, PDB code: 1THM). For details, see text.