Figures & data
Figure 1. Overexpression of WIND homologs promotes cell dedifferentiation without exogenous phytohormones. (A–C) 14-d-old wild-type (WT, A) and 14, 21-d-old (B, C, respectively) 35S:WIND4 seedlings grown on phytohormone-free MS medium. 35S:WIND4 seedlings display severe morphological defects and callus induction. (D) 60-d-old callus excised from 35S:WIND4 seedlings. (E, F) 8-d-old XVE-WIND1 seedlings germinated on phytohormone-free MS medium without (-) (E) or with (+) 10 mM 17b-estradiol (F). (G, H) Cross sections of cotyledon epidermal cells in 9-d-old XVE-WIND1 plants. XVE-WIND1 seedlings were germinated without (G) or with (H) 10 mM 17b-estradiol. Cellular boundaries are visualized by propidium iodide (PI) and cross sections are generated from confocal Z-stacks. Scale bars, 2 mm (A, C, D), 500 mm (B, E, F), 20 mm (G, H).
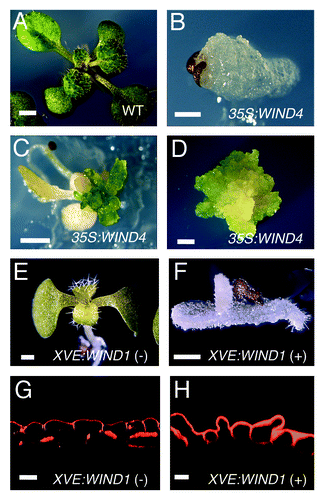
Figure 2. WIND1 expression is induced locally at the wound site. (Left panels) Light micrograph of 4-d-old intact ProWIND1:GFP plants (A) and 6-d-old ProWIND1:GFP plants incubated for 48 h (h) after root dissection (B). (C) Magnified view of ProWIND1:GFP root segments marked with a white box in (B). (Right panels) The WIND1 promoter activity visualized by the GFP expression. Strong GFP signals are seen at both ends of the dissected roots as indicated by arrows. Scale bars, 2 mm (A, B, C)
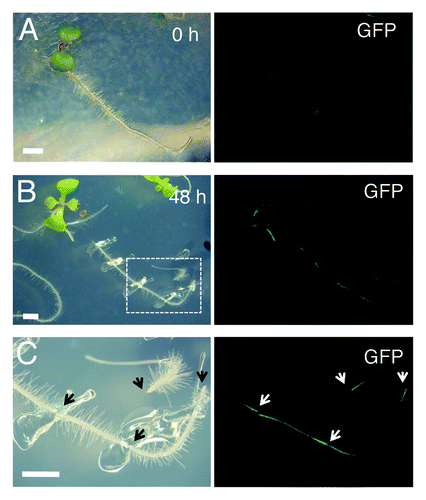
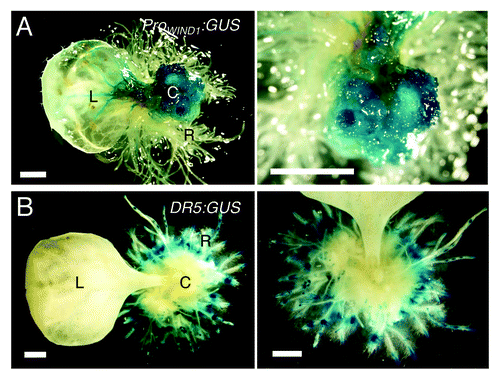
Figure 3. WIND1 expression persists in dedifferentiated callus cells. (Left panels) Rosette leaves of 14-d-old plants carrying the ProWIND1:GUS (A) and DR5:GUS (B) constructs were cultured on 1 µM NAA containing MS medium for 30 d. (Right panels) Magnified view of the NAA-induced callus and roots. The ProWIND1:GUS signal is strong in callus cells while the DR5:GUS signal is not found in callus and detectable remarkably in roots in contact with the medium. L: leaf; C: callus; R: regenerated roots from callus. Scale bars, 1 mm (A, B)