Figures & data
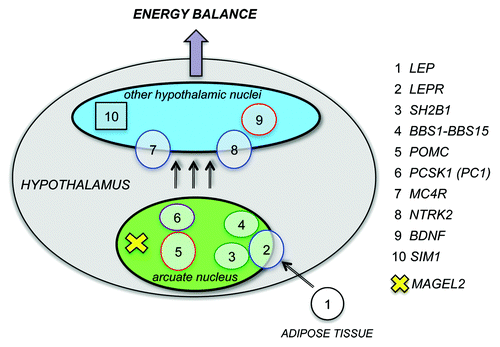
Figure 1. Genes mutated in childhood obesity act in the hypothalamus to regulate energy balance. Mutations that cause rare monogenic or syndromic forms of childhood obesity have been identified in at least 10 different genes, listed on the right. The proteins encoded by these genes act in the hypothalamus (gray) and participate in the leptin - melanocortin pathway that regulates appetite and body weight. MAGEL2 is one of the genes inactivated in Prader-Willi syndrome, a rare genetic disorder that causes childhood-onset severe obesity. Mercer et al. showed that Magel2, the murine ortholog of MAGEL2, is essential for the leptin-mediated responses of a specific set of neurons, those that express POMC in the arcuate nucleus of the hypothalamus.