Figures & data
Figure 1. DM1-associated RNA spliceopathy and the expression of splicing regulators in the CNS. (A) Representative splicing analysis of candidate genes in the frontal cortex and brainstem of DM1 patients (n = 4–5) and non-DM controls (n = 2) by RT-PCR (Table S1). The alternative exons are indicated on the right of each panel. The higher molecular product results from the amplification of the transcript that includes the alternative exon (+); the lower band does not include the alternative exon (-). (B) Western blot analysis of MBNL1 and MBNL2 in the frontal cortex and brainstem of wild-type animals (Table S1), following long migration times to increase resolution and separation of protein isoforms. β-Actin was used as loading control. (C) Representative splicing analysis of alternative exons of Grin1/Nmdar1, Mapt/Tau, Mbnl1 and Mbnl2 by RT-PCR (Table S1) in the spinal cord of one-month-old DMSXL homozygous mice and wild-type controls (n = 3, per genotype). Newborn splicing profiles (P1) were determined in a cDNA pool prepared from three wild-type animals. Notably, DMSXL spinal cord exhibited increased exclusion of both Grin1/Nmdar1 exon 5 and Mapt/Tau exon 10, as well as a mild increase in the inclusion of both Mbnl1 exon 7 and Mbnl2 exon 7. (D) Western blot analysis of splicing regulators throughout wild-type embryonic development (E12.5, E14.5, E18.5), in newborn (P1), postnatal day 8 (P8), and adult mice aged one (M1), four (M4) and 10 (M10) months (Table S2). Protein extracts from three individual animals were pooled for each developmental stage, electrophoresed and analyzed in three independent assays. Representative western blots are shown for frontal cortex and brainstem. Over-exposed of MBNL1 and MBNL2 western blots are shown to confirm low protein expression during embryonic stages and at P1. (E) Western blot analysis of hnRNP H in the frontal cortex and brainstem of DMSXL and wild-type animals at one month of age (Table S2), when missplicing dysregulation is more pronounced. Protein extracts from three individual animals of each genotype were pooled. β-Tubulin was used as loading control.
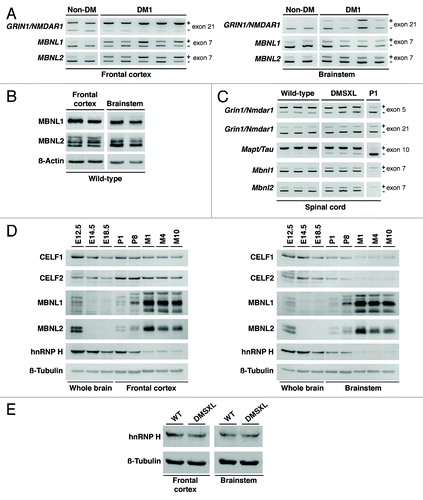
Figure 2. Expression, phosphorylation, and alternative splicing of RAB3A and SYN1. (A) Quantification of RAB3A steady-state levels and SYN1 phosphorylation throughout wild-type brain embryonic development (E10.4, E12.5, E14.5, E18.5), in newborn (P1), postnatal day 8 (P8), and adult mice aged one (M1), four (M4) and 10 (M10) months (Table S2). Frontal cortex protein extracts from three individual animals were pooled for each developmental stage and analyzed in two independent western blot assays (only one is shown). The graphs on the right represent the mean RAB3A and SYN1 steady-state levels (± SD) throughout the development and aging of mouse frontal cortex, as well as mean SYN1 phosphorylation on amino acid residues serine-9 (S9) and serine-553 (S553). (B) Representative RT-PCR splicing analysis of RAB3A exon 2 and the 5′ end of SYN1 exon 13 in the frontal cortex of DM1 (n = 9) and non-DM individuals (n = 4), as well as in DMSXL and wild-type mice at four months of age (n = 6, each genotype) (Table S1). The 5′ end of exon 2 of Rab3A is alternatively spliced only in mouse, and included in the analysis. The graphs show the mean inclusion ratio (± SEM) of each alternative exon or alternative 5′ end.
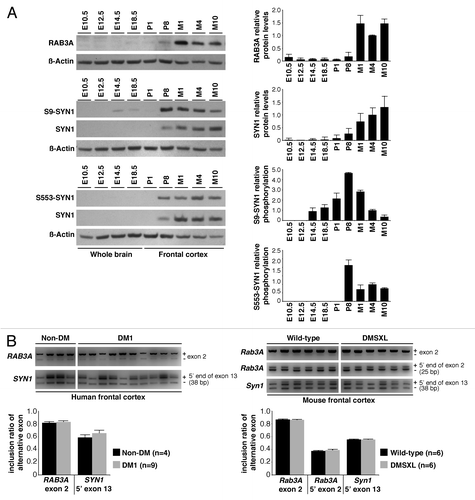
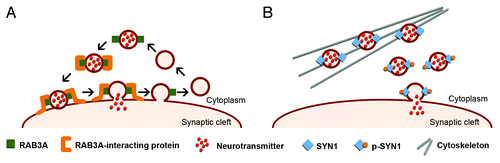
Figure 3. Schematic representation of the role of RAB3A and SYN1 in the dynamics of synaptic vesicles. (A) RAB3A is an abundant vesicle-associated protein that regulates synaptic efficiency. The reported functions of RAB3A in exocytosis range from docking and fusion of the synaptic vesicle to its subsequent recycling. RAB3A functions through a variety of interactions with multiple effector proteins. The upregulation of RAB3A likely perturbs the highly regulated vesicle dynamics and release, affecting neurotransmission and synaptic function in DM1. (B) SYN1 is expressed in mature neurons, where it associates with the cytoplasmic surface of synaptic vesicles. SYN1 regulates the supply of synaptic vesicles available for exocytosis by binding to both vesicles and actin cytoskeleton in a phosphorylation-dependent manner. Under resting conditions, non-phosphorylated SYN1 attaches synaptic vesicles to the actin cytoskeleton. Synaptic stimulation induces SYN1 phosphorylation that facilitates vesicle dissociation from cytoskeleton and potentiates exocytosis. Abnormal SYN1 hyperphosphorylation in DM1 likely dysregulates neuronal exocytosis and vesicle release.