Figures & data
Figure 1. Toxic events caused by mutant CUG repeats in DM1 cells and possible therapeutic approaches for their correction. Mutant CUG repeats cause three toxic molecular events: (1) sequestration of MBNL1, (2) higher levels of CUGBP1, leading to elevation of the active form of CUGBP1 (CUGBP1ACT), and (3) elevation of active GSK3β, which reduces cyclin D3 and converts a portion of CUGBP1ACT to CUGBP1REP. Thus, both CUGBP1 forms are elevated in DM1. Reduced MBNL1 and increased CUGBP1ACT lead to deregulation of mRNA splicing, translation, and stability. Futhermore, increased CUGBP1REP may reduce mRNA translation in stress granules. Taken together, these molecular changes lead to myotonia, weakness, and muscle atrophy. Administration of GSK3β inhibitors reduces DM1 muscle histopathology, weakness, and myotonia similar to degradation of mutant CUG repeats by AONs.
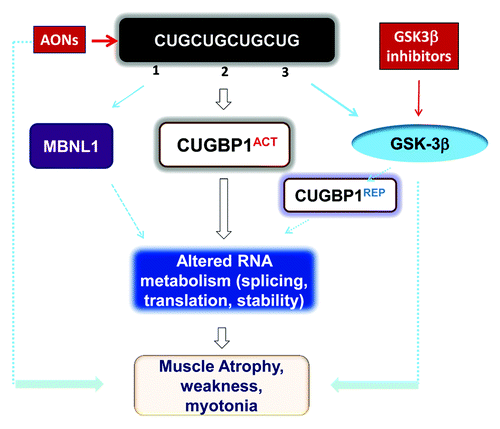
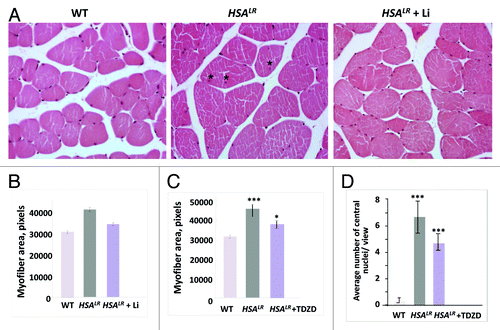
Figure 2. Correction of muscle histopathology in HSALR mice treated with GSK3 inhibitors, namely lithium and TDZD-8. (A) Hematoxylin and eosin staining of muscle sections (gastrocnemius) from age-matched 6-mo-old WT, untreated HSALR mice, and lithium-treated HSALR mice. Internal nuclei in the muscle of untreated HSALR mice are indicated by asterisks. (B) Lithium reduces myofiber size variability in HSALR mice. Myofiber area was compared in gastrocnemius from age-matched 6-mo-old WT mice and HSALR mice untreated and treated for 2 weeks with lithium. The y-axis shows the average myofiber area in pixels. p < 3.36 × 10−16 (untreated HSALR mice vs. WT mice); p < 1.10 × 10−7 (treated vs. untreated HSALR mice). (C) TDZD-8 treatment reduces myofiber size variability in HSALR muscle. Myofiber area was increased in 4-mo-old HSALR mice relative to WT mice (***p < 5.88 × 10−6). The myofiber area was reduced in HSALR mice after TDZD-8 treatment. *p < 0.02677 (treated HSALR mice vs. untreated). (D) Treatment of HSALR mice with TDZD-8 reduces the number of central nuclei in the skeletal muscle (gastrocnemius) of HSALR mice. The number of central nuclei was counted in six randomly selected 20 × views and the average values are shown. The average number of central nuclei per view was increased in HSALR mice (***p < 4.616 × 10−8) relative to WT mice. However, treatment with TDZD-8 reduced the number of central nuclei. ***p < 0.000387 (treated HSALR mice vs. untreated).