Figures & data
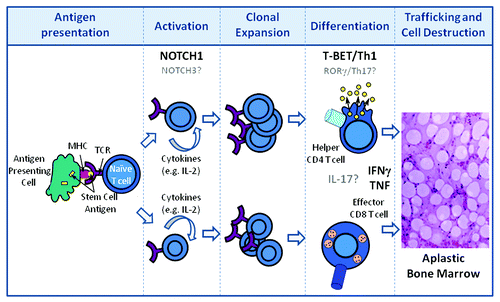
Figure 1. Disease progression in aplastic anemia. Following antigen presentation of HSC self- or neoantigen(s) by antigen presenting cells during the initiation phase of the immune response, T cells become activated and release growth factors (IL-2) which result in the clonal expansion of CD4 and CD8 T cells. Expanded T cells differentiate into Th1 helper (CD4) and cytolytic (CD8) T cells. CD4 and CD8 T cells traffic to the bone marrow and produce pro-inflammatory cytokines which are directly toxic to HSCs. NOTCH1 contributes to Th1 differentiation through its direct regulation of T-BET. HSC destruction is also mediated directly by effector CD8 T cells through mechanisms involving FAS-FASL interactions as well as cytolytic granule release and, indirectly, through loss of supportive stromal cells through “by-stander” effects of the pro-inflammatory microenvironment. Putative contributions of NOTCH3, rorγ, and IL-17 are indicated in gray.