Figures & data
Figure 1. Structures of NusG (A) and RfaH (B). All structures are shown in ribbon representation whereas the central β-sheet of the NTD is highlighted in dark colors. The flexible linker is depicted as black line. Black arrows indicate interaction partners of the respective domain. In (B) the transformation of RfaH from an autoinhibited into an active state is shown as its CTD refolds from an all-α into an all-β state. The figure was created using PyMOL.Citation36 PDB IDs: 2K06, NusG NTD; 2JVV, NusG CTD; 2OUG, RfaH; 2LCL, RfaH CTD
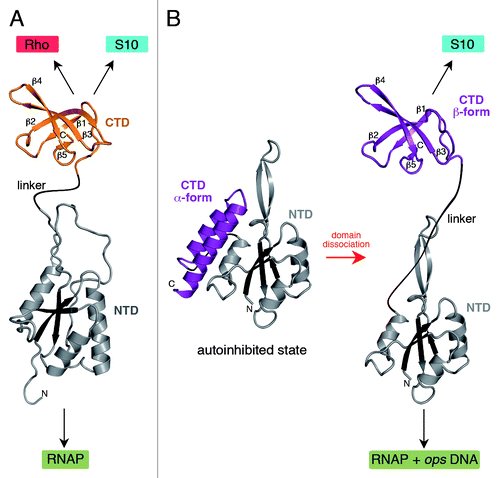
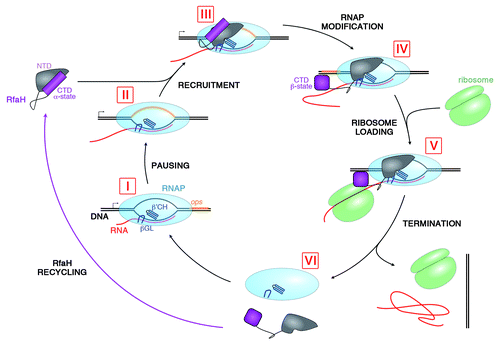
Figure 2. Lifecycle of RfaH. During transcription of RfaH-controlled operons RNAP pauses at the ops element in the leader region (I and II). RfaH, in its autoinhibited form, interacts with the ops DNA via its NTD; the domains then separate, allowing RfaH NTD to bind RNAP (III). Together with β’CH and βGL, RfaH NTD forms a clamp around the nucleic acids chains to increase the processivity of RNAP. The freed RfaH CTD completely refolds from its α-helical form into a β-barrel (IV). The transformed CTD binds S10, recruiting a ribosome to the mRNA, and translation commences (V). After termination of translation, the transcription elongation complex dissociates (VI). The CTD of released RfaH folds back into its α-form and RfaH is recycled into its autoinhibited state, ready for the next round of recruitment.