Figures & data
Figure 1. Localization of three different type I TA loci on the E. coli K-12 genome. hok/sok (pink), ldr/rdl (blue) and symE/symR (green) loci are shown. Asterisks show genes that are clearly degenerated or relics.
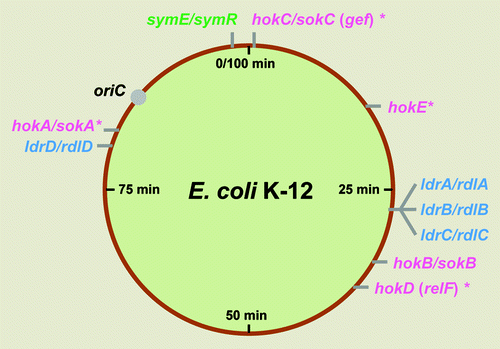
Figure 2. Genetic organization of hokB/sokB, ldrD/rdlD, and symE/symR type I TA modules of E. coli K-12. (A) The hokB/sokB locus is located between cybB and trg at 32.1 min (). This system contains all of the regulatory elements as described for hok/sok system in plasmid R1, such as fbi (foldback inhibition) element, tac (translational activation) element, ucb (upstream complementary box) promoter sequences, Shine-Dalgarno sequences and an overlapping reading frame mokB (mediation of killing). The mokB reading frame is out-of-frame with hokB and terminates 38 nt upstream of hokB. (B) The ldrD/rdlD locus is located between bcsG and yhjV at 79.7 min (). A second open reading frame ldrX, noted by Gerdes and Wagner,Citation16 that overlaps with ldrD as in-frame, thus they share the same translational termination codon. It is predicted that RdlD RNA regulates ldrD translation by regulating ldrX translation. (C) The symE/symR locus is located between restriction-modification related genes mcrB and hsdS at 98.7 min (). The sym E promoter has a LexA binding site and is strongly induced by DNA damaging agents. SymR is encoded opposite the 5′ untranslated region (UTR) of symE, and base pairing can extend over the Shine-Dalgarno sequence as well as the initiation codon of symE.
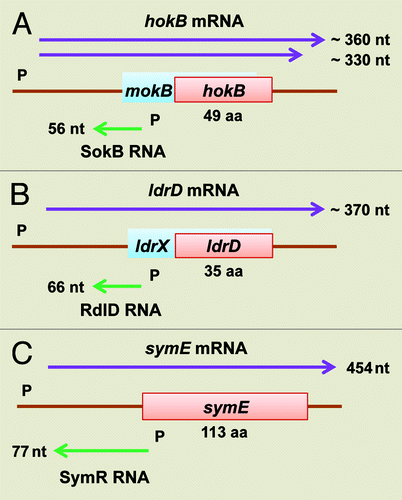
Figure 3. Model for SymE synthesis. SOS-induced symE gene is repressed at three levels by (1) the LexA repressor (transcriptionally), (2) the SymR antisense RNA (post-transcriptionally and/or translationally) and (3) the Lon protease (post-translationally). Other as-yet unknown factors such as ribonucleases and chaperon proteins could be involved in the modulation of SymE synthesis. Endogenous levels of the SymE protein might play a role in degrading particular RNA damaged concomitantly with DNA.
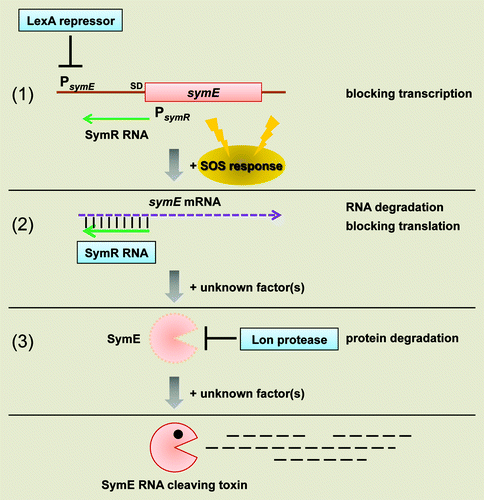
Figure 4. Model for timing of SymE synthesis during the SOS response. The SOS genetic network consists of more than 40 genes in E. coli that carry out diverse functions in response to DNA damage, including nucleotide excision repair, homologous recombination, translesion DNA replication, and cell division arrest. The network is controlled by the LexA repressor that downregulates itself and the expression of the other SOS genes, but the peak timing of the induced protein levels seems to be different. SymE protein synthesis may occur at the late stage of the SOS response but before cell lysis. It is suggested that SymE promotion of RNA cleavage may be important for ribosome rescue by recycling of RNAs damaged under SOS-inducing conditions.
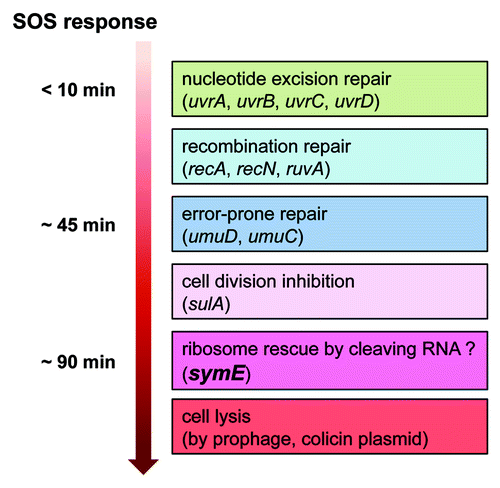
Figure 5. Multiple amino acid sequence alignments. (A) Hok proteins from E. coli K-12, E. coli O157, and plasmid R1. (B) Ldr proteins from E. coli K-12, Salmonell typhimurium LT2, Salmonella typhi CT18, and Citrobacter freundii. Identical amino acids are boxed, and similar amino acids are indicated by an asterisk at the bottom. The similarity of amino acids was determined by the following rules: L = I = M = V = f = W = A, K = R = H, D = E = Q = N, G = A = S, t = V, A = V and f = Y = H = W. + shows C-terminal positive charged residues. The black line above the aligned amino acids indicates a putative trans-membrane α-helical domain predicted by a computer program (SOSUI: http://bp.nuap.nagoya-u.ac.jp/sosui/).
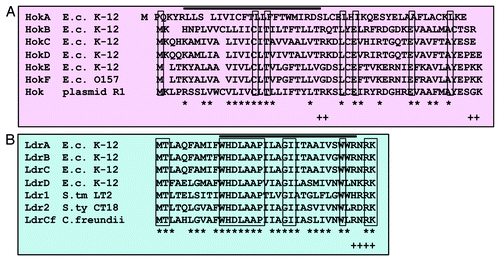
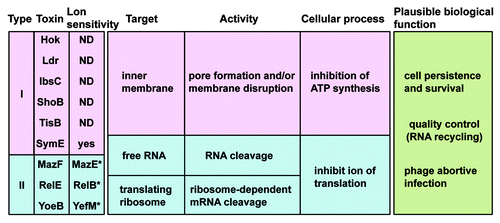
Figure 6. Summary of the type I and II toxins. The targets, types of activity, and cellular processes that are affected by the endogenous toxin expression levels need to be examined in more detail. Asterisk denotes paired antitoxin gene.