Figures & data
Figure 1 Morphology of wr spermatozoa. Spermatozoa were collected from the cauda epididymes of 35 days-old wr (A–F) and wild-type (G) mice. Nuclei were counterstained with DAPI. Note the rounded (globic, (A–E) and globoid, (F)) heads lacking an acrosome and the deformed, mislocalized mitochondrial sheath (C–E) of the wr sperm. Scale bar, 5 µm.
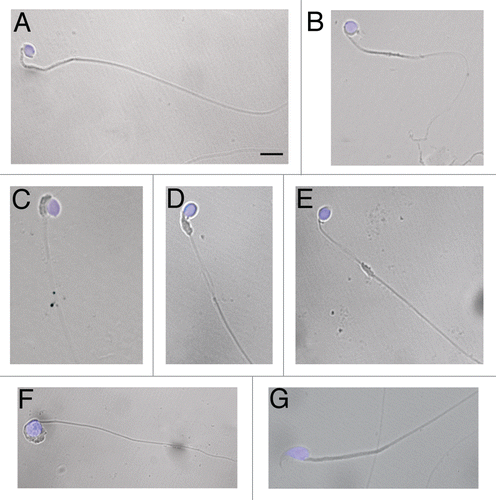
Figure 2 Histology of 35 (A–C), 44 (D–F) and 77 (G–I) days-old testis. Representative cross sections of the seminiferous epithelium of wr (left and middle columns) and wild-type (right column) mice. Black arrowheads and arrows point to round spermatids and elongated spermatids/spermatozoa, respectively. White arrows point to the clumping of round-headed spermatozoa next to be released (D) and to aggregates of residual bodies containing clumped spermatids (G). Asterisk signs the loosening/loss of Sertoli-germ cell contacts at the basal compartment in wr (H) versus wild type littermate (I). Note the round heads/nuclei and vacuolated cytoplasm of wr spermatids/spermatozoa in contrast to the hook-shaped heads devoid of remnants of cytoplasm of the wild-types. Scale bar, 10 µm.
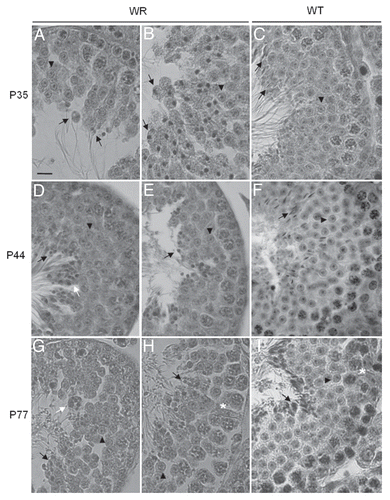
Figure 3 Loosening of anchoring junctions between Sertoli cells and germ cells at the basal compartment in 77 days-old wr testis. (A) Electron micrograph showing a representative cross-section of a seminiferous tubule from 77 days-old wild-type testis. This micrograph also illustrates the intimate relationship between the seminiferous epithelium (composed of Sertoli cells and germ cells) and the basement membrane (BM) of the tunica propria. (B) A magnification of (A) showing the intimate relationship between a Sertoli cell (SC) and a preleptotene spermatocyte (PS). (C) Enlargement of inset shown in (B): black arrowheads indicate the intact germ cell-Sertoli cell junctional complex. (D, F and H) Electron micrographs illustrating an altered architecture of the seminiferous epithelium at the basal compartment in 77 days-old wr testis. Abnormal and extensive intercellular spaces (asterisks) are found between Sertoli cells and spermatocytes (SP) and Sertoli cells and spermatogonia (SG). (E) Magnified view of the boxed area shown in (D). (G) Magnified view of the boxed area shown in (F). (I) Magnified view of the boxed area shown in (H) illustrating a desmosome-like junction, partially still intact, between a spermatogonium and a Sertoli cell. Scale bars, 2 µm (A, F and H); 1 µm (B–E and G); 0.5 µm (I).
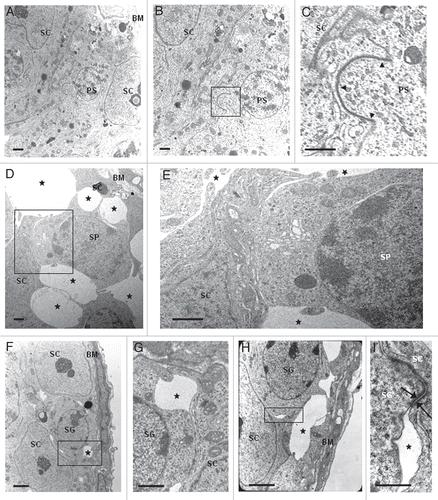
Figure 4 PAS-hematoxylin staining of wild-type (A–F) and wr (G–I) mouse testis to detect different phases of acrosomogenesis. Representative cross-sections of seminiferous tubuli are shown. Stages of the seminiferous epithelial cycle are marked with Roman numerals. PAS-positive (pink) proacrosomal granules, acrosomal caps and acrosomes are indicated by arrows. In contrast to the wild type (D–F), no evident acrosomal caps and acrosomes were detected, independently from the acrosomogenetic step, in the wr testis (G–I). PAS-staining resulted only in punctuate spots (arrows) scattered into the wr spermatid cytoplasm. Scale bar, 10 µm.
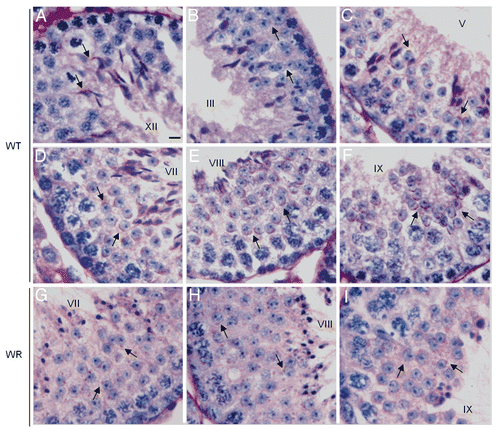
Figure 5 Localization of UBPy in testis from wr mice. Sections of wr (A and B) and wild-type (C and D) testes at stage VII of the seminiferous epithelial cycle were immunofluorescently labeled with antibodies to UBPy (red); DAPI (blue) was used to stain nuclei. Note the diffuse punctuate staining of UBPy in wr spermatids; in particular, the enlargement in (B) shows that no spermatid at step 7 exhibits the brightly fluorescent UBPy-immunostaining of the acrosomal cap characteristic of the wild-type step 7 spermatids (enlargement in D). Scale bars, 20 µm (A), 26 µm (C) and 10 µm (B and D).
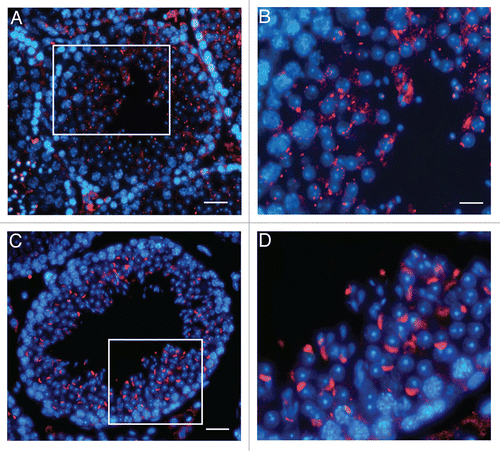
Figure 6 Localization of Vps54(L967Q) by immunofluorescence analysis in wr testis at different ages (P35, P44 and P77). Stages of the seminiferous epithelial cycle are marked with Roman numerals. Sections of wr (left and middle columns)) and wild-type littermate (right column) testes were labeled with antibodies to Vps54 (green); DAPI (blue) was used to stain nuclei. Compare the Vps54(L967Q) and Vps54 immunostainings. Vps54(L967Q) marks punctuated vesicles scattered within the spermatid cytoplasm, independently from the acrosomogenetic step. These vesicles do not develop into a polarized acrosomal cap (A and B, G and H) and acrosome (D and E) as, on the contrary, it occurs for the Vps54-labeled vesicles in the respective stages of the wild-type littermate spermatids/spermatozoa (C, I and F, respectively). Scale bars, 10 µm (A, D and G); 5 µm (B, C, F and I); 6 µm (E and H).
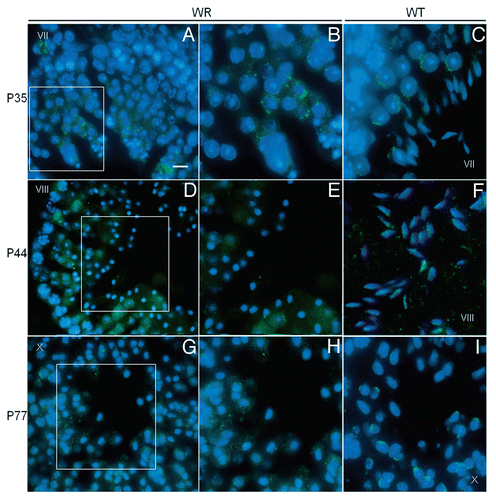
Table 1 Testis weights, epididymal sperm counts and seminiferous tubule diameters of age-matched wobbler and wild-type male mice