Figures & data
Figure 1. A schematic molecular model of filamin A illustrating its role as a F-actin cross-linker. The actin-binding domain (ABD) of filamin A is located at its N-terminus, which is followed by the 9–15 immunoglobulin (Ig) repeats, constituting the Rod 1, which is capable of binding one F-actin filament. The hing region (H1), the Rod 2 region and the H2 region are also shown. The 24 Ig repeat is the dimerizing domain where two subunits of filamin A are dimerized via the two 24 Ig repeats through non-covalent interactions. The shaded “gray” area between the two Rod 2 domains of two filamin A subunits is the region where filamin A interacts with its binding partners, such as integrins, Rho, Rac, Cdc42, ROCK, Pak1, FiGAP, dopamine receptor and others.
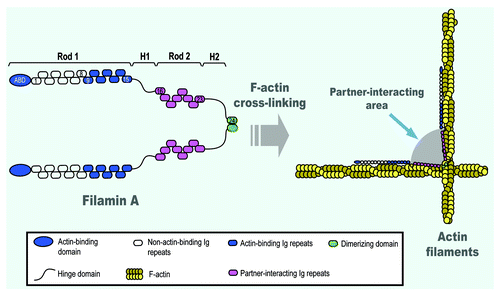
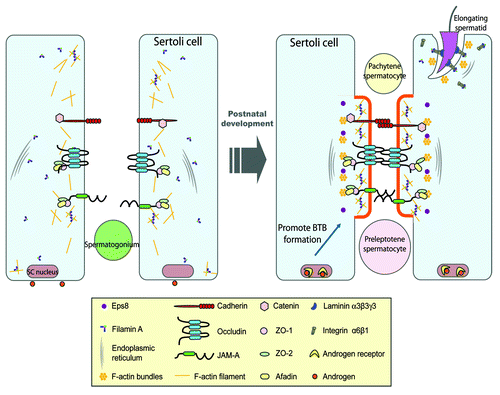
Figure 2. A schematic drawing illustrating the role of filamin A in the assembly of a functional BTB during postnatal development. In immature rat testes, cell adhesion protein complexes (e.g., occludin-ZO-1, cadherin-catenin, JAM-A-ZO-1) cannot be recruited to the BTB site to assemble the functional TJ-permeability barrier (see left panel). At age 17–25 d postpartum, the expression of filamin A increases, the functional filamin A recruits the assembly of actin filament network at the BTB site, which in turn, recruits cell adhesion protein complexes. This process is facilitated by androgen, which induces cross-linking of F-actin filaments mediated by filamin A to form rigid scaffold underneath cell membrane, which can lead to membrane protrusion at cell-cell interface to facilitate adhesion formation (see right panel). Also, during spermiogenesis, the assembly of cell adhesion protein complexes (e.g., integrin-laminin) at the Sertoli-spermatid interface, namely the apical ectoplasmic specialization (apical ES), is likely facilitated by the recruitment of integrins to the apical ES via interactions between integrins (e.g., α6-integrin, β1-integrin) and filamin A (right panel).