Figures & data
Figure 1. Schematic representation of vertebrate Semaphorins and their receptors, Plexins and Neuropilins. Each semaphorin contains one sema and one PSI domains. Class III Semaphorins are secreted and possess a C-terminus basic-rich domain. They signal through Plexin (Plx) A and Neuropilins (NRP), except for Sema3E that only required PlxD1. Class IV Semaphorins are transmembrane proteins and operate through PlxD1 and PlxB. Sema5A is also transmembrane and characterized by thrombospondin repeats, and binds to PlxB3. Transmembrane class VI Semaphorins use PlxA, while GPI-anchored Sema7A signals through PlxC1.
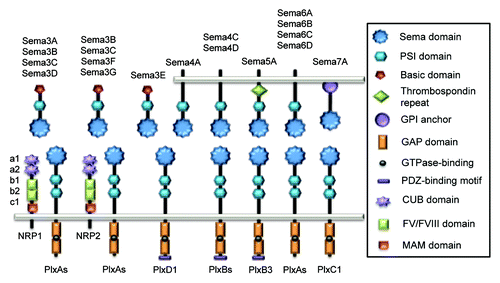
Figure 2. Impact of Semaphorins on the organization of epithelial cell-cell junctions. Specific and distinct adhesion proteins structure the epithelial cell junctions: tight junctions (JAM, occludin and claudins) and adherens junctions (nectin and E-cadherin) are linked to actin cytoskeleton and multiple intracellular adaptators. Upon exposure to Sema3A, 3B, 3C and 3F, cell adhesion can be strengthened, while Sema3E, 4D and 7A provoked dramatic cell-cell junction remodeling, which may ultimately favor tumorigenesis.
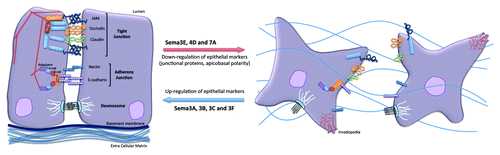
Figure 3. Impact of Semaphorins on the organization of endothelial cell-cell junctions. Endothelial cell-cell junctions are organized around intertwined adherens (nectin and VE-cadherin) and tight junctions (JAM, occludin and claudins). Other non-junctional adhesive proteins, such as PECAM, an endothelial marker, and N-cadherin, which anchors pericytes, might collectively contribute to the endothelial barrier properties. Semaphorins exert either pro- or anti-barrier actions on the endothelial junctions, which in turn alter vascular properties including permeability and angiogenesis.
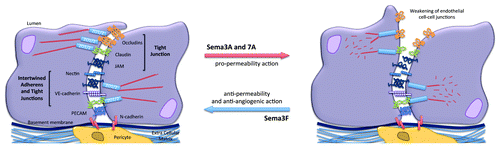
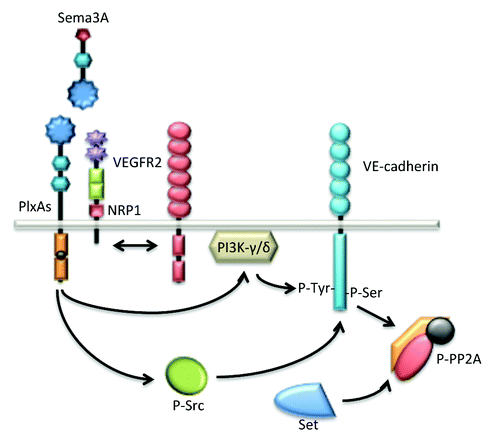
Figure 4. Sema3A enhances endothelial permeability through VE-cadherin destabilization. Sema3A directly augments endothelial permeability by the mean of PlxA1 and NRP1 receptors. Further downstream signaling relies on the activation of the kinase activities bear by PI3K-γ/δ and Src. These pathways culminate at the phosphorylation and destabilization of VE-cadherin. Interestingly, in quiescent endothelial cells, VE-cadherin is associated with the phosphatase PP2A, a mechanism possibly involved in locking the adherens junctions. In this model, Src-directed PP2A phosphorylation and inactivation contribute in turn to VE-cadherin destabilization upon Sema3A challenge.