Figures & data
Figure 1. AQP5−/− mice are protected against cigarette-smoke induced emphysema. (A) Mean Linear Intercept (MLI) was significantly increased in WT mice exposed to 6 mo of CS compared with air-exposed WT mice and air-exposed AQP5−/− mice. n = 7–10 per group, p < 0.006. (B)Surface to volume ratio (S/V) was significantly reduced in WT mice exposed to 6 mo of CS compared with air-exposed WT mice and air-exposed AQ5−/− mice, n = 7–10 per group, *p < 0.02. (C) Representative histologic H&E lung sections (20x) demonstrate airspace size for various exposure and WT or AQP5−/− mice groups.
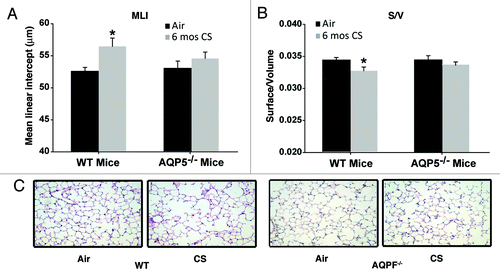
Figure 2. AQP5−/− mice demonstrate reduced epithelial permeability. (A) We assessed permeability in trachea by efflux of Evans’ blue dye (EBD). Trachea from AQP5−/− mice had reduced EBD efflux at baseline. Trachea from WT mice exposed to 1 d of CS had increased EBD efflux compared with trachea from WT mice exposed to air and compared with trachea from both groups of AQP5−/− mice. n = 5 per group, ^p < 0.05 compared with all other groups,*p < 0.05 compared with WT air trachea group. (B) We determined the ratio of BAL EBD to Lung EBD. EBD ratio was increased only in WT mice exposed to 4 weeks of CS exposure, in comparison to air-exposed WT mice and air-exposed AQP5−/− mice. n = 5 per group, p = 0.001. (C) We quantified E-cadherin by lung immunofluorescence. CS-exposed WT mice had a decrease in E-cadherin expression compared with air-exposed WT mice, ^p < 0.05. Both air-exposed and CS-exposed AQP5−/− mice had increased E-cadherin compared with both WT groups (*p < 0.05), and had similar E-cadherin expression to each other. n = 3.
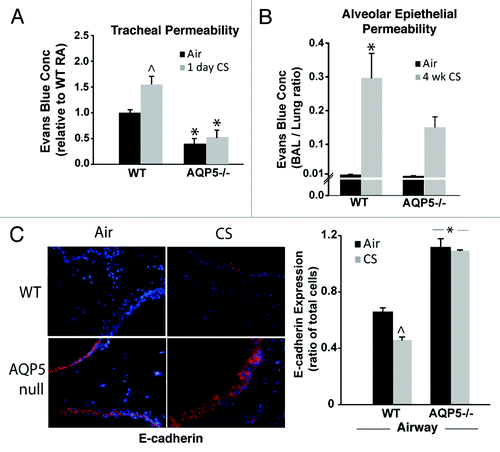
Figure 3. AQP5 alters CS-induced effects on type I and II pneumocyte presence. (A) Using flow cytometry, there was a similar percentage of CD326+ cells, a pan-epithelial marker, between WT and AQP5−/− mice after 6 mo of CS exposure. n = 7–10. (B) Among CD326+ cells, there was a significant reduction in the percentage of T1α positive cells, a type I AEC marker, in AQP5−/− mice exposed to 6 mo CS compared with similarly exposed WT mice. n = 8–10, p = 0.013. A histogram representing percent positivity and mean fluorescence intensity is shown.(C) AQP5−/− mice exposed to CS had increased SPC expression by immunofluorescence (IF), a type II AEC marker, compared with CS-exposed WT mice. (D) AQP5−/− mice exposed to 6 mos CS express higher levels of SPC compared with WT mice exposed to 6 mos CS in homogenized lung; densitometry was expressed as a ratio of SPC to actin. E. Dot plot measuring the percentage of Ki-67+ cells among CD326+ cells demonstrates similar cluster and median values between WT and AQP5−/− mice after 6 mo of CS.
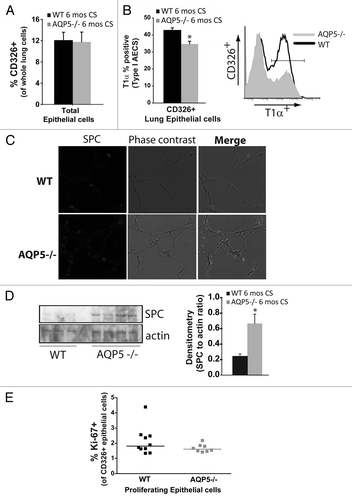
Figure 4. Absence of AQP5 mitigates alveolar neutrophil abundance after sub-acute and chronic cigarette smoke. (A) WT and AQP5−/− mice had an increase in total bronchoalveolar (BAL) cells after 6 mo of CS exposure, n = 7–10 per group, *p < 0.05. (B) WT mice had a ~10-fold increase in BAL neutrophils after 6 mo of cigarette smoke exposure, n = 7–10 per group, *p < 0.05 compared with all other groups. (C) WT mice and AQP5−/− mice demonstrate a significant increase in BAL macrophages and BAL lymphocytes after 6 mo of CS compared with WT air-exposed mice; macrophage increase in CS-exposed AQP5−/− mice was not significant compared with air-exposed AQP5−/− mice, n = 8–10 per group, *p < 0.05 compared with both air-exposed groups, ^p < 0.05 compared with WT air-exposed mice. (D) After sub-acute (4-week) CS exposure, WT mice have a ~5-fold increase in alveolar neutrophils compared with all other groups. n = 7–10 per group, p < 0.05. (E) AQP5−/− mice demonstrate a significant increase in BAL macrophages only when compared with air-exposed WT mice after 4-week CS exposure. n = 7–10 per group, p < 0.05. (F) Using adenovirus, we increased AQP5 expression by immunoblot (+AQP5) in A549 cells; CS exposure did not alter AQP5 expression. We measured neutrophil migration across an A549 monolayer. At baseline, there was no AQP5-mediated difference in PMN migration. After CS exposure, there was a significant increase in PMN migration across A549 cells expressing AQP5 (+AQP5) compared with A549 without AQP5 expression (control), n = 4, p < 0.05.
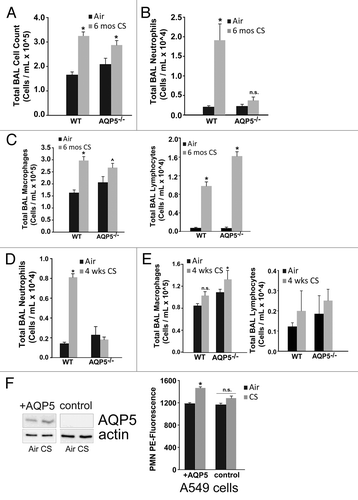
Figure 5. AQP5 alters neutrophil signaling for migration across the alveolar epithelial layer. (A) Using flow cytometry, CD11b mean fluorescence intensity (MFI) on BAL neutrophils was significantly reduced in CS-exposed AQP5−/− mice compared with CS-exposed WT mice and air-exposed AQP5−/− mice. n = 7–10 per group, *p < 0.05. CD11b MFI on BAL macrophages is similarly increased in CS-exposed WT and AQP5−/− mice. n = 7–10 per group, *p < 0.05. (B) Among total lung cells, the percentage of lung interstitial neutrophils (Gr-1+) is not increased in WT or AQP5−/− mice after 6 mo of CS exposure, but lung neutrophil expression of CD11b is significantly increased in WT and AQP5−/− mice exposed to chronic CS. n = 7–10 per group, *p < 0.05
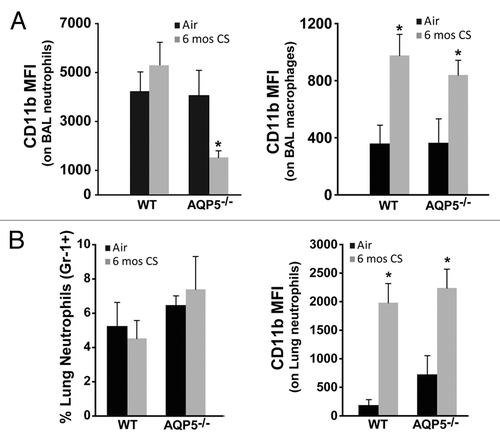
Figure 6. AQP5 alters epithelial expression for ICAM-1 after CS. We measured ICAM-1 immunofluorescence (labeled red) in lung sections after air or chronic CS exposure, and enumerated it as a ratio of total cells (defined by labeled nuclei). WT mice exposed to CS had increased airway (top graph) and alveolar (bottom) epithelial cell ICAM-1 expression compared with air-exposed WT mice. AQP5−/− mice exposed to CS had similar ICAM-1 expression compared with air-exposed AQP5−/− mice and less than WT mice exposed to CS. n = 3, *p < 0.05.
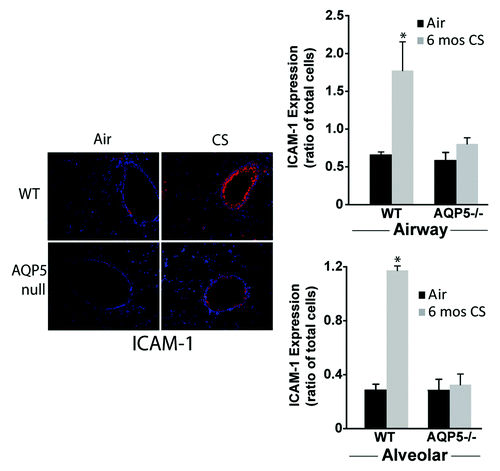
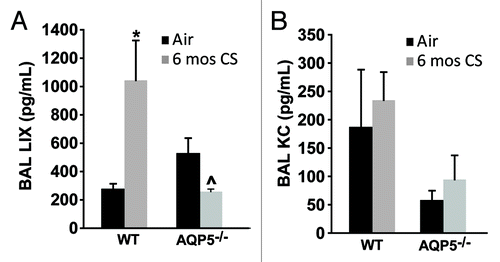
Figure 7. The absence of AQP5 blunts epithelial-derived chemokine signal for PMN recruitment. (A) BAL LPS-induced chemokine (LIX), primarily epithelial-derived, was significantly increased in CS-exposed WT mice compared with air-exposed WT mice, and significantly reduced in CS-exposed AQP5−/− mice compared with CS-exposed WT mice and air-exposed AQP5−/− mice. n = 4–5, *p < 0.05, ^p = 0.029. (B) BAL keratinocyte chemokine (KC), both epithelial and non-epithelial cell derived, was similar between WT and AQP5−/− mice. n = 4–5.